Classification of Colloids
Table of Content |
Classification of colloids based on Physical state
|
Dispersed Phase |
Dispersion Medium |
Name |
Typical example |
|
Solid |
Liquid |
Sol |
Gold sol, Mud, Fe(OH)3 sol, |
|
Solid |
Solid |
Solid sol |
Gems, Ruby glass, Minerals |
|
Solid |
Gas |
Aero sols |
Smoke (Carbon in air) Volcanic dust |
|
Liquid |
Solid |
Gel |
Curd, Cheese, Jellies |
|
Liquid |
Liquid |
Emulsion |
Milk, water in benzene, cream |
|
Liquid |
Gas |
Liquid aero sol |
Clouds, fog (water in air) mist |
|
Gas |
Solid |
Solid foam |
Lava, Pumica |
|
Gas |
Liquid |
Foam |
Froth on beer , whipped cream |
-
 A colloidal system in which the dispersion medium is a liquid or gas is called sols. They are called hydrosols or aqual sols. If the dispersion medium is water. When the dispersion medium is alcohol or benzene, they are accordingly called alcohols or benzosol.
A colloidal system in which the dispersion medium is a liquid or gas is called sols. They are called hydrosols or aqual sols. If the dispersion medium is water. When the dispersion medium is alcohol or benzene, they are accordingly called alcohols or benzosol. -
Colloidal systems in which the dispersion medium is air are called aerosols.
-
Colloids in which the dispersion medium is a solid are called gels, e.g. chese etc. They have a more rigid structure. Some colloids, such as gelatin, can behave both as a sol and a gel. At high temperature and low concentration of gelatin, the colloids is hydrosol. But at low temperature and high gelatin concentration, the hydrosol can change into a gel.
Classification of colloids based on nature of interaction between dispersed phase and dispersion medium
- Lyophilic sols: These tem lyophilic means liquid-loving (i.e. solvent loving). Certain substances have an affinity for certain liquids and readily form colloidal dispersions with them.
 The substances which when mixed with a suitable liquid (dispersion medium) readily form colloidal solutions are called lyophilic colloids or intrinsic colloids and the sols thus formed are called lyophilic sols.
The substances which when mixed with a suitable liquid (dispersion medium) readily form colloidal solutions are called lyophilic colloids or intrinsic colloids and the sols thus formed are called lyophilic sols.
When water is used as the dispersion medium, such colloids are termed as hydrophilic colloids and their colloidal dispersion in water are known as hydrophilic sols.
Arabic gum, gelatin, albumin, starch etc. are some common examples of lyophilic colloids. Gum sol, starch sol, sols of proteins in water, sols of polymers in organic solvents etc. is some examples of lyophilic sols.
Lyophilic sols are stable and do not get precipitated easily. In fact, they are self stabilized, i.e. they do not require any stabilizing agent to preserve them. An important characteristic of these sols is that if dispersed phase is separated from dispersion medium (say by evaporation), the dispersed phase can again be brought in the sol state simply by mixing it with the dispersion medium. This is why hydrophilic sols are also known as reversible sols.
- Lyophobic sols: The term lyophilic means liquid-hating (i.e. solvent-hating). The substances which do not pass much affinity for the dispersion medium and do not readily pass into the sol state when mixed with the medium are called hydrophobic colloids or extrinsic colloids.Their sols are prepared by using special techniques and they are referred to as hydrophobic sols.
Sols of metals e.g. gold sol, platinum sol etc. and the sols of insoluble substances such as metal sulphides and oxides are some examples of lyophilic sols.
Lyophilic sols are relatively less stable as compared to lyophilic sols.They are easily precipitated (or coagulated) on addition of small amounts of electrolytes, by heating or agitation. Moreover, the precipitated dispersed phase cannot be brought back into the sol state by simply mixing it with the dispersion medium. This is why hydrophobic sols are also known as irreversible sols.
The lyophobic sols need stabilizing agents to keep them in the sol form for a longer time.
The important points of difference between the lyophilic sols and lyophobic sols are summarized in the table given below:
Classification of Colloids Based on Type of Particles of the Dispersed Phase
We have already seen that the colloidal particles present in a colloidal system have size lying in the range 1nm-100nm. Depending upon how different substances forming colloidal solution acquire the size of particles in this range, colloidal solutions may be classified into the following three categories. 
-
Multimolecular Colloids: When a large number of atoms or small molecules (having diameters of less than 1nm) of a substance combine together in a dispersion medium to form aggregates having size in the colloidal range, the colloidal solutions thus formed are called multimolecular colloids. The species (atoms or molecules) constituting the dispersed particles in multimolecular colloids are held together by Vander Waals’ forces.
The gold sol, sulphur sol etc. are some examples of multimolecular colloids. A gold sol may contain particles of various size composed of several atoms of gold. Similarly, sulphur sol consists of particles containing about a thousand of S8molecules. -
Macromolecular Colloids: Certain substances form large molecules whose dimensions are comparable to those of colloidal particles. Such molecules have very high molecular masses and are termed as macromolecules. When such substances are dispersed in suitable dispersion medium, the resulting colloidal solutions are known as macromolecular colloids. Thus, in macromolecular colloids, the dispersed particles are themselves large molecules having very high molecular masses.
Most of the lyophilic sols are macromolecular colloids. For example colloidal dispersion of naturally occurring macromolecules such as starch, proteins, gelatin, cellulose, nucleic acids etc. are macromolecular colloids. Synthetic polymers such as polyethylene, polypropylene, synthetic rubber etc. also form macromolecular colloids when dispersed in suitable solvents. -
Associated Colloids (Micelles): Associated colloids are those colloids which behave as normal strong electrolytes at low concentrations but exhibit colloidal properties at higher concentrations due to the formation of aggregated particles. The aggregated particles thus formed are called micelles.
-
The associated colloids are usually formed by surfactants (surface active agents) like soaps and synthetic detergents. These agents form micelles when present in solution at a concentration greater than critical micellization concentration (CMC). The formation of a micelle can be understood by taking the example of soap solution as described below.
Micelle Formation in Soap Solutions
The commonly used soaps are sodium or potassium salts of higher fatty acids such as palmitic acid (C15H31COOH), stearic acid (C17H35COOH) etc.
Rfer to the follwoing video for cleansing action of soap.
The most commonly used washing soap is sodium stearate, C17H35COONa. In general, soap can be represented as RCOONa, where R represents a long chain alky1 group. When dissolved in water, soap ionizes to give RCOO– and Na+ ions.
The RCOO– ion consists of two parts – long hydrocarbon chain R and the polar group -COO– as shown diagrammatically. The hydrocarbon residue R is hydrophobic, i.e. water repelling, whereas the -COO- group is hydrophilic, i.e. water loving. Therefore, RCOO– ion orients itself in such a way that -COO- end dips in water and the group R stays away from water.
The -COO- groups of different RCOO- ions tend to stay away from one another due to like charges,whereas the R-groups try to approach each other to form other to form a bunch. This leads to the formation of a micelle as shown in the figure. 
Thus a soap micelle is a negatively charged colloids particle in which the negatively charged -COO– groups are arranged in a spherical manner at the surface, while the hydrocarbon chains point towards the centre.
The -COO– groups present at the surface of the micelle get surrounded by Na+ ions which tend to drag the micelle in the bulk of the solution. A micelle may contain as many as 100 molecules or more.
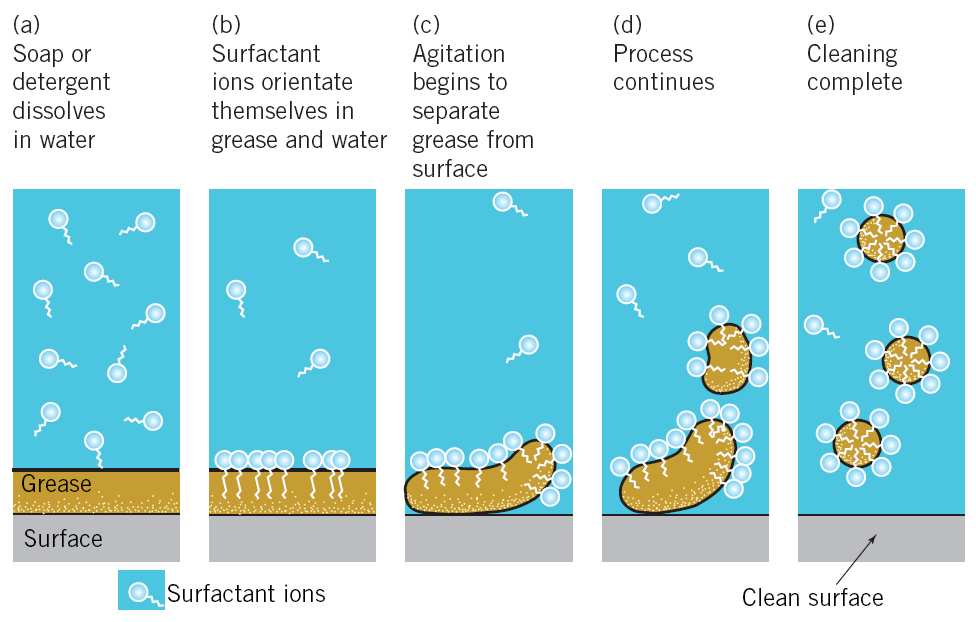
The cleansing action of soaps: Soaps are used for cleaning dirty clothes. Clothes become dirty due to the deposition of dust and oily or greasy substances. Water is not capable of wetting oily or greasy substances. However, the hydrocarbon residue R of the soap anion (RCOO–) can do so. When a dirty cloth is dipped into a soap solution, the hydrocarbon residue R of RCOO– ion dissolves the oily or greasy dirt and encapsulates it to form a micelle.
When the cloth is rinsed with water, the micelles carrying the oily or greasy dirt get washed away.
The cleaning action of detergents such as sodium laury sulphate, CH3 (CH2)11SO4Na+ or sulphonates of long chain hydrocarbons is similar to that of soaps. In case of detergents, the polar groups are sulphate (–SO4) or sulphonate (-SO3) groups.

Question 1: Cheese is a
a. sol
b. gel
c. foam
d. emulsions
Question 2: Which of the following colloid is an example of solid foam?
a. gem
b. rubby
c. glass
d. lava
Question 3: Colloidal syems in which the dispersion medium is air are called
a. aerosols
b. benzosols
c. aquasols
d. micelles
Question 4: Certain substances form large molecules whose dimensions are comparable to those of colloidal particles. Such molecules have very high molecular masses and are termed as
a. macromolecular colloids
b. micromolecular colloids
c. associated colloids
d. micelles
|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
b |
d |
a |
a |
Related Resources
-
You can also refer to syllabus of chemistry for IIT JEE
-
Click here access the past year papers of IIT JEE
-
Click here for Enzyme Catalysis
To read more, Buy study materials of Surface Chemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free