Commercial Production of Chemicals
Table of Content |
|
|

 As we discussed earlier electrolysis has a great application in manufacturing of large number of chemicals. We are going to discuss some of them in details in this topic.
As we discussed earlier electrolysis has a great application in manufacturing of large number of chemicals. We are going to discuss some of them in details in this topic.
Manufacture of Sodium
Sodium is obtained on large scale by two processes:
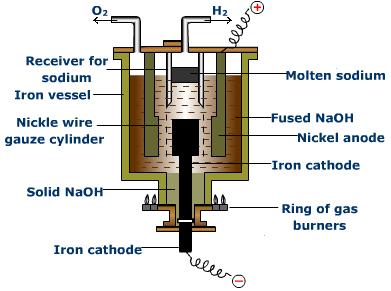
Castner's Process
-
In this process, electrolysis of fused sodium hydroxide is carried out at 330°C using iron as cathode and nickel as anode: 2NaOH
 2Na+ + 2OH
2Na+ + 2OH -
At cathode : 2Na+ + 2e → 2Na
-
At anode: 4OH- → 2H2O + O2 + 4e
-
During electrolysis, oxygen and water are produced.
-
Water formed at the anode gets partly evaporated and is partly broken down and hydrogen is discharged at cathode : H2O
 H+ OH-
H+ OH- - At cathode: H+ + 2e → 2H H2
|
Castner's process |
||
|
At cathode |
At anode |
Overall |
|
2Na+ + 2e → 2Na & |
4OH- → 2H2O + O2 + 4e |
2NaOH |
Down's Process
-
Now-a-days sodium metal is manufactured by this process.
-
It involves the electrolysis of fused sodium chloride containing calcium chloride and potassium fluoride using iron as cathode and graphite as anode at about600oC.
-
NaCl
 Na+ + Cl-
Na+ + Cl- -
At cathode: Na+ + e → Na
-
At anode: 2Cl- → Cl2 + 2e
The electrolysis of pure NaCl presents the following difficulties:(i) The fusion temperature of NaCl is high, i.e., 803°C. At this temperature both sodium and chlorine are corrosive. (ii) Sodium forms a metallic fog at this temperature. |
-
To remove above difficulties, the fusion temperature is reduced to 600°C by adding CaCl2 and KF.
-
This is a cheaper method and chlorine is obtained as a by-product. The sodium obtained is of high purity (about 99.5%).
|
Down's process |
|
|
At cathode |
|
|
Na+ + e → Na |
2Cl-→ Cl2 + 2e |
Sodium Hydroxide (Caustic soda), NaOH
Caustic soda is manufactured by the electrolysis of aqueous solution of sodium chloride in an electrolytic cell.
Principle
-
A sodium chloride solution contains Na+, H+, Cl- and OH- ions.
- NaCl
 Na+ + Cl-
Na+ + Cl- - H2O
 H+ + OH-
H+ + OH- -
On passing electricity, Na+ and IF ions move towards cathode and CI- and OH" ions move towards anode.
-
The discharge potential of H+ ions is less than Na+ions, thus hydrogen ions get discharged easily and hydrogen is liberated. Similarly, at anode CI- ions are easily discharges as their discharge potential is less than that of OH- ions. Cl2 gas is, therefore, liberated at anode.
-
The solution on electrolysis becomes richer in Na+ and OH- ions.
-
Since chlorine reacts with sodium hydroxide solution even in the cold forming sodium chloride and sodium hypochlorite, it is necessary that chlorine should not come in contact with sodium hydroxide during electrolysis. 2NaOH + Cl2 → NaCl + NaCIO + H2O
-
To overcome this problem, the anode is separated from the cathode in the electrolytic cell either by using a porous diaphragm or by using a mercury cathode.
Porous Diaphragm Process (Nelson Cell Process)
Nelson cell consists of a perforated steel tube lined inside with asbestos. The tube acts as a cathode (Fig. 12.19).
-
It is suspended in a steel tank.
-
A graphic rod dipped in sodium chloride solution serves as anode.
-
On passing electric current, chlorine is liberated at the anode and let out through the outlet.
-
Sodium ions penetrate through the asbestos and reach the cathode where hydrogen and OH- ions are formed by reduction of water.
-
Sodium ions combine with OH- ions to form NaOH which is collected in the outer tank while hydrogen is drawn off through the outlet.
-
The steam blown during the process keeps the electrolyte warm and helps to keep perforation clear.
-
At Cathode 2H2O+2e
 H2 + 2OH- & Na+ OH-
H2 + 2OH- & Na+ OH-  NaOH
NaOH -
At anode 2Cl- → Cl2 + 2e
-
The solution containing NaOH and Nacl as impurity is taken out and evaporated to dryness.
|
Nelson cell process |
|
|
At cathode |
At anode |
|
2H2O+2e |
2Cl-→ Cl2 + 2e |
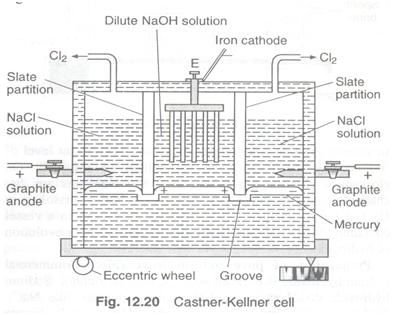
Castner-Kellner Cell
-
This is the common cell in which mercury is used as cathode.
-
The advantage to using Hg as a cathode is that the discharge potential of Na+ ions is less than that of H+ ions. Na+ ions get discharged on mercury and the sodium so deposited combines with mercury to form sodium amalgam.
-
The cell consists of a large rectangular trough divided into three compartments by slate partitions which do not touch the bottom of the cell but dipping in mercury.
-
The mercury can flow from one compartment into other but the solution kept in one compartment cannot flow into other.
-
Sodium chloride solution is placed in the two outer compartments and a dilute solution of sodium hydroxide in the inner compartment.
-
Two graphite electrodes which act as anodes are fixed in the outer compartments and a series of iron rods fitted in the inner compartment acts as cathode.
-
Mercury in the outer compartments acts as cathode while in the inner compartment it acts as anode by induction. The cell is kept rocking with the help of an eccentric wheel.
-
When electricity is circulated, sodium chloride in the outer compartments is electrolysed.
-
Chlorine is evolved at the graphite anode while Na+ ions are discharged at the Hg cathode.
-
The liberated sodium forms amalgam with mercury: NaCl
 Na+ + Cl-
Na+ + Cl- -
At anode 2Cl- + 2e → Cl2
-
At cathode Na+ + e → Na
-
Na + Hg → Amalgam
-
The sodium amalgam thus formed comes in the inner compartment due to rocking. Here, the sodium amalgam acts as the anode and iron rods acts as cathode.
-
At anode : Na-amalgam → Na+ + Hg + e
-
At cathode : 2H2O + 2e → H2 + 2OH-
-
The concentrated solution of sodium hydroxide (about 20%) is taken out from the inner compartment and evaporated to dryness to get solid NaOH.
|
At Anode |
At Cathode |
|
2Cl- + 2e → Cl2 Na+ + e → Na |
Na+ + e → Na 2H2O + 2e → H2 + 2OH- |
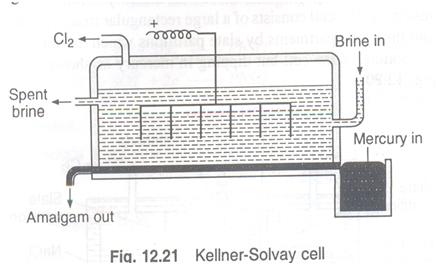
Kellner-Solvay Cell
-
This is the modified cell. This cell has no compartments.
-
The flowing mercury acts as cathode.
-
A number of graphite rods dipping
-
A constant level of sodium chloride solution is maintained in the cell.
-
On electrolysis chlorine gas is librated and Na+ ions are discharged ay cathode (mercury).
-
Sodium discharged dissolves in Hg and forms amalgam.
-
This amalgam flows out in a vessel containing water. sodium hydroxide is formed with evolution of hydrogen.
Preparation of Pure Sodium Hydroxide
-
Commercial sodium hydroxide is purified with the help of alcohol.
-
Sodium hydroxide dissolves in alcohol while impurities like NaCl, Na2CO3, Na2SO4, etc., remain insoluble.
-
The alcoholic filtrate is distilled.
-
The alcohol distills off while pure solid sodium hydroxide is left behind.
Manufacture of Aluminium
-
Aluminium is manufactured from pure bauxite ore by electrolysis.
-
The bauxite ore usually contains impurities such as iron oxide, silica, etc.
-
These impurities are first removed by the application of the following methods in order to get pure alumina, i.e., pure bauxite ore.
(a) Hall's process
(b) Baeyer's process
(c) Serpeck's process.
Electrolytic Reduction of Pure Alumina
The electrolysis of pure alumina faces two difficulties:(i) Pure alumina is a bad conductor of electricity, (ii) The fusion temperature of pure alumina is about 2000°C and at this temperature when the electrolysis is carried of the fused mass, the metal formed vapourises as the boiling point of aluminium is 1800°C. |
-
The above difficulties are overcome by using a mixture containing alumina, cryolite (Na3AlF6) and fluorspar (CaF2) in the ratio of 20 : 60 : 20. The fusion temperature of this mixture is 900°C and it is a good conductor of electricity.
-
The electrolysis is carried out in an iron box lined inside with gas carbon which acts as cathode. The anode consists of carbon rods which dip in the fused mixture of the electrolyte from above. The fused electrolyte is covered with a layer of coke (Fig. 12.22).
-
The current passed through the cell serves two purposes:
-
Heating of the electrolyte: The temperature of the cell is automatically maintained at 900-950°C.
-
Electrolysis: On passing current,aluminium is discharged at cathode.
-
Aluminium being heavier than the electrolyte sinks to the bottom and is tapped out periodically from a tapping hole.
-
Oxygen is liberated at anode. It attacks the carbon rods forming CO and CO2.
-
The process is continuous. When the concentration of the electrolyte decreases, the resistance of the cell increases.
-
This is indicated by the glowing of a lamp placed in parallel. At this stage more of alumina is added.
-
The exact mechanism of the electrolysis is not yet known.
-
Two concepts have been proposed.
-
First concept: AlF3 from cryolite ionizes as: AlF3
 Al3+ + 3F-
Al3+ + 3F- - Al3+ ions are discharged at cathode and F- ions at anode.
|
At anode |
At cathode |
|
Al3+ + 3e → Al |
2F- → F2 + 2e |
-
The liberated fluorine reacts with alumina to form AlF3 and O2. The oxygen attacks the carbon anodes to form CO and CO2.
-
Al2O3 + 3F3 → 2AlF3 + O2
-
2C + O2→ 2CO
-
C + O2 → CO2
-
Anodes are replaced frequently.
-
Second concept: Alumina (Al2O3) ionizes as: Al2O3 → Al3+ + AlO33-
-
At cathode: Al3+ + 3e → Al
-
At anode: AlO3- → 2Al2O3 + 3O2 + 12e
-
Thus, the overall chemical reaction taking place during electrolysis is: 2Al2O3→ 4Al + 3O2
-
Aluminum of 99.8% purity is obtained from this process.
Refining of Aluminum by Hoope's Electrolytic Method
-
Aluminum is further purified by Hoope's process. The electrolytic cell consists of an iron box lined inside with carbon.
-
The cell consists of three layers which differ in specific gravities. The upper layer is of pure aluminium which acts as cathode.
-
The middle layer consists of a mixture of the fluorides of Al, Ba and Na. The lowest layer consists of impure aluminium which acts as anode.
-
The middle layer works as electrolyte.
-
The graphite rods are dipped in pure aluminium and Cu-Al alloy rods at the bottom of impure aluminium work as conductors.
-
On electrolysis, aluminium is deposited at the cathode from the middle layer and an equivalent amount of aluminium is taken up by the middle layer from the bottom layer (impure aluminium).
-
Therefore, aluminium is transferred from bottom to the top layer through middle layer while impurities are left behind. Aluminium thus obtained is 99.98%.
Isolation of Fluorine
-
Fluorine presented many difficulties in its isolation.
-
It remained a difficult problem in chemistry for many years and after a hard labour of many chemists for about 75 years it could be isolated finally by Moissan in 1886.
-
The reasons for its late discovery were its high reactivity and non-conducting nature of hydrofluoric acid.
-
Fluorine attacked the material of the vessels used for its isolation. Carbon vessel was attacked with formation of CF4 and platinum vessel was reduced to chocolate powder.
-
The vessels of other metals were also affected. Platinum and carbon could not be used as electrodes.
-
Another difficulty experienced was that when the electrolysis of aqueous hydrofluoric acid was carried out, hydrogen and oxygen (ozone) were obtained and when anhydrous hydrofluoric acid was tried it was found to be a bad conductor of electricity.
-
Moissan finally solved the problem and isolated fluorine by the electrolysis of anhydrous hydrofluoric acid in the presence of potassium hydrogen fluoride using Pt-Ir alloy vessel at -23°C.
Modern Methods of Isolation
-
In modern methods, fluorine is prepared by electrolysis of a fused fluoride (usually potassium hydrogen fluoride, KHF2).
-
The electrolytic cells are made of copper, nickel or monel metal.
-
The anode is generally of graphite and the fluorine set free contains some carbon tetrafluoride.
Reactions in the Electrolytic Cell
The following precautions should be taken in the preparation of fluorine:
-
The electrolyte must be completely dry. In presence of moisture, the evolved fluorine reacts with moisture to form 02 and 03.
-
The parts of the apparatus which come in contact with fluorine must be free from oil and grease.
-
The vessel in which fluorine is collected should also be absolutely dry.
-
The gas must be made free from HF before storing by passing through sodium fluoride (NaF), otherwise HF will attack the vessel.
[Note: HF is more corrosive and reactive than fluorine.]
Dennis Method
-
The electrolytic cell used in this method consists of a V-shaped copper tube (5 cm in diameter) fitted with copper caps.
-
Graphite electrodes through these caps are sealed and insulated in the tube by bakelite cement which is not. affected by fluorine.
-
The cen is covered with an insulating layer of asbestos cement over which is wound a resistance wire for electrical heating.
-
The tube is thickly lagged to prevent the loss of heat.
-
The electrolyte consists of fused potassium hydrogen fluoride which has already been dried for 48 hours at 130°C.
-
The electrolyte is kept in fused state by electrical heating externally. For electrolysis, a current of 5 amperes and 12 volts is used. On electrolysis fluorine is liberated at anode.
-
To make the liberated fluorine free from HF vapours, it is passed through copper U-tubes containing sodium fluoride: NaF + HF ---> NaHF2
-
The following difficulty is experienced in this method: The liberated fluorine at anode does not escape fast enough due to narrow exit.
-
The escape is further hindered due to frothing in the electrolyte. There are, thus, chances of mixing of H2 and F2 which may result in explosion.
-
To avoid this, a modified apparatus has been devised by Whytlaw-Gray.
|
Whytlaw-Gray method |
|

Question 1:
Which of the following reactions take place at anode in Castner's process?
a. 4OH- → 2H2O + O2 + 4e
b.2C + O2→ 2CO
c. AlO3- → 2Al2O3 + 3O2 + 12e
d. 2Cl- + 2e → Cl2
Question 2:
Sodium is manufactured by
a. Whytlaw-Gray method
b. Castner's process:
c. Dennis method:
d. Hoope's electrolytic method:
Question 3:
Nelson cell process is used for manufacturing of
a. NaIOH
b. NaCl
c. HCl
d. Al2O3
Question 4:
Caustic soda is manufactured by the electrolysis of aqueous solution of
a. NaOH
b.NaHCO3
c.Na2CO3

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
b |
a |
d |
Related Resources
-
You can also refer to the Syllabus of IIT JEE
-
Click here for the Past year Papers of IIT JEE
-
Refer to Solved Examples on Electrochemistry
To read more, Buy study materials of Electrochemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

 2Na+ + 2OH
2Na+ + 2OH




.gif)

