Batteries
Table of Content |

 Any cell or Battery (more than one cells connected in series) that we use daily as a source of electricity in basically a galvanic cell in which chemical energy of spontaneous redox reactions is converted into electrical energy.
Any cell or Battery (more than one cells connected in series) that we use daily as a source of electricity in basically a galvanic cell in which chemical energy of spontaneous redox reactions is converted into electrical energy.
An ideal battery should have following desirable characters..
-
It should be reasonably light
-
It should be compact
-
Its voltage should not vary much during its life i.e., period of use.
There are mainly two types of batteries which we use today
-
Primary Batteries
-
Secondary batteries
Primary Batteries or Voltaic Cells (Dray Cells)
-
In this tye of cell, once the chemicals have been consumed, further reaction is not possible.
-
After use over a period of time battery becomes dead and cannot be reused again
-
It cannot be regenerated by reversing the current flow through the cell using an external direct current source of electrical energy.
-
The most common example of this type is dry cell.
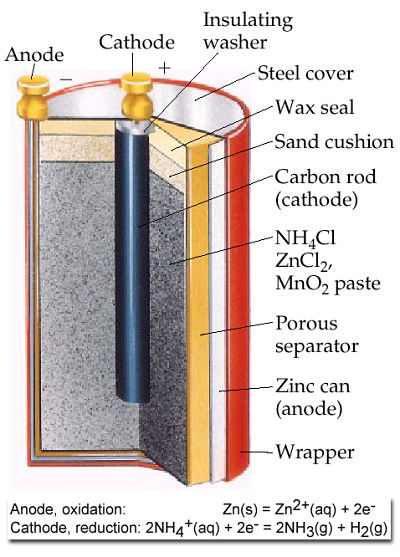
Dry Cell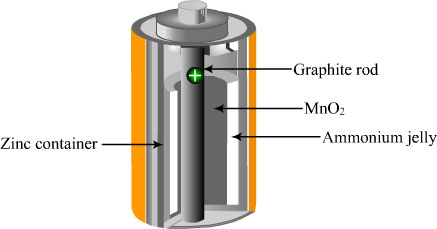
-
The container of the dry cell is made of zinc which also serves as one of the electrodes.
-
The other electrode is a carbon rod in the centre of the cell.
-
The zinc container is lined with a porous paper.
-
A moist mixture of ammonium chloride, manganese dioxide, zinc chloride and a porous inert filler occupy the space between the paper lined zinc container and the carbon rod.
-
The cell is sealed with a material like wax.
-
As the cell operates, the zinc is oxidised to Zn2+
-
Anode reaction: Zn → Zn2+ + 2e-
-
The electrons are utilized at carbon rod (cathode) as the ammonium ions are reduced.
-
Cathode Reaction: 2NH4++2e →2NH3 + H2
-
The cell reaction is: Zn+ 2 NH4+ → Zn2+ + 2NH3 + H2
-
Hydrogen is oxidized by MnO2 in the cell: 2MnO2 + H2 →2MnO(OH)
-
Ammonia produced at cathode combines with zinc ions to form complex ion. Zn2+ + 4NH3 →[Zn(NH3)4]2+
-
Ecell is 1.6 volt
Chemical Reactions in Dry Cell |
||
|
Reaction at Anode |
Reaction at Cathode |
Cell Reaction |
|
Zn →Zn2+ + 2e- |
2NH4++2e- → 2NH3 + H2 |
|
Alkaline dry cell
-
Alkaline dry cell is similar to ordinary dry cell. It contains potassium hydroxide.
-
Ecell is 1.5 volt.
-
The reaction in alkaline dry cell are:
Chemical Reactions in Alkaline Dry Cell |
||
|
Reaction at Anode |
Reaction at Cathode |
Cell Reaction |
|
Zn + 2OH- → Zn(OH)2 + 2e- |
2MnO2 + 2H2O + 2e- → 2MnO(OH) + 2OH- |
Zn + 2MnO2 + 2H2O → Zn(OH)2 + 2MnO(OH) |
Secondary Batteries or Voltaic Cells (Lead Storage Battery)
-
The cell in which original reactants are regenerated by passing direct current from external source, i.e., it is re-charged, is called secondary cell.
-
Lead storage battery is the example of this type.
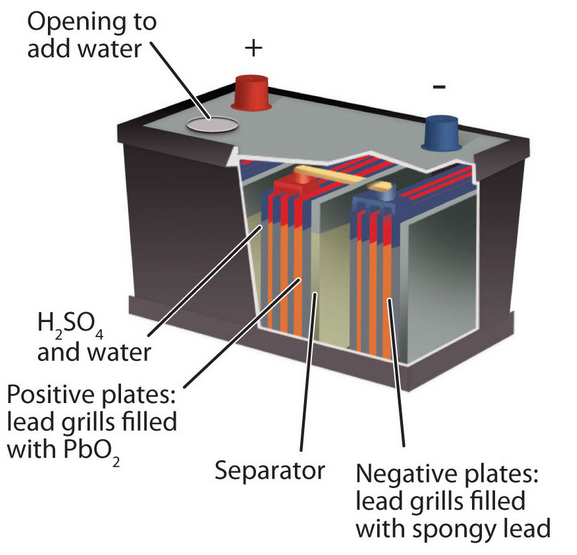
Lead Storage Battery
-
Lead storage battery consists of a group of lead plates bearing compressed spongy lead, alternating with a group of lead plates bearing leaf dioxide, PbO2.
-
These plates are immersed in a solution of about 30% H2SO4.
-
When the cell discharge; it operates as a voltaic cell.
-
The spongy lead is oxidized to Pb2+ ions and lead plates acquire a negative charge.
-
Anode Reaction: Pb → Pb2+ + 2e-
-
Pb2+ ions combine with sulphate ions to form insoluble lead sulphate, PbSO4, which begins to coat lead electrode.
-
The electrons are utilized at PbO2 electrode.
-
Cathode Reaction: PbO2 + 4H+ + 2e-→ Pb2+ +2H2O
-
Precipitation: Pb2+ + SO42- → PbSO4
-
Overall cell reaction is: Pb + PbO2 + 4H+ + 2 SO42-→ 2PbSO4 + 2H2O
-
Ecell is 2.041 volt.
-
When a potential slightly greater than the potential of battery is applied, the battery can be re-charged. 2PbSO4 + 2H2O → Pb + PbO2 + 2H2SO4
-
After many repeated charge-discharge cycles, some of the lead sulphate falls to the bottom of the container, the sulphuric acid concentration remains low and the battery cannot be recharged fully.
Chemical Reactions in Lead Storage Battery |
||
|
Reaction at Anode |
Reaction at Cathode |
Cell Reaction |
|
Pb → Pb2+ + 2e- |
PbO2 + 4H+ + 2e-→ Pb2+ +2H2O |
|
Fuel Cell
-
Fuel cells are another means by which chemical energy may be converted into electrical energy.
-
The main disadvantage of a primary cell is that it can deliver current for a short period only.
-
This is due to the fact that the quantity of oxidising agent and reducing agent is limited.
-
But the energy can be obtained indefinitely from a fuel cell as long as the outside supply of fuel is maintained.
One of the Examples is the Hydrogen-Oxygen Fuel Cell
-
The cell consists of three compartments separated by a porous electrode.
-
Hydrogen gas is introduced into one compartment and oxygen gas is fed into another compartment.
-
These gases then diffuse slowly through the electrodes and react with an electrolyte that is in the central compartment.
-
The electrodes are made of porous carbon and the electrolyte is a resin containing concentrated aqueous sodium hydroxide solution.
-
Hydrogen is oxidised at anode and oxygen is reduced at cathode.
-
This type of cells are used in space-crafts. Fuel cells are efficient and pollution free.
-
The overall cell reaction produces water. The reactions which occur are:?
Chemical Reactions in Fuel Cell |
||
|
Reaction at Anode |
Reaction at Cathode |
Cell Reaction |
|
[H2(g) + 2OH-(aq) → 2H2O(l) + 2e- |
O2(g) + 2H2O(l) + 4e- → 4OH-(aq) |
2H2(g) + O2(g) → 2H20(l) |

Question 1:
Which of the following equations represents the oxidation reaction of a secondary cell?
a. Pb → Pb2+ + 2e-
b. Pb2+ + SO42- → PbSO4
c. Zn+ 2 NH4+ → Zn2+ + 2NH3 + H2
d. Zn → Zn2+ + 2e-
Question 2:
In which of the following alternatives, once the chemicals have been consumed, further reaction is possible.
a. Dry Cell
b. Alkaline Dry Cell
c. Mercury cell
d. Lead Storage Battery
Question 3:
EMF of lead storage battery is.
a. 2.041 V
b. 12.43 V
c. 1.92 V
d. 5.324 V
Question 4:
Which of the following substances is not present in dry cell?
a. Ammonium chloride,
b. Manganese dioxide,
c. Zinc chloride

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
d |
a |
d |
Related Resources
-
Refer to the Syllabus of Chemistry for IIT JEE
-
Look here for the Sample Papers for IIT JEE
-
You can also refer to Kohlarusch’s Law
To read more, Buy study materials of Electrochemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More