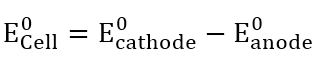Galvanic Cells
Table of Content |
|
|
Terms to Know
 Electrochemistry - The branch of science that deals with interconversion of chemical energy into electrical energy and vice versa.
Electrochemistry - The branch of science that deals with interconversion of chemical energy into electrical energy and vice versa.
Oxidation - Loss of electron(s) by an atom, ion or molecule is called Oxidation / Increase in oxidation number.
Reduction - Gain of electron(s) by an atom, ion or molecule is called Reduction / Decrease in oxidation number.
LIO-GIR – Loss is oxidation-gain is reduction of electrons.
Oxidation Number - The charge possessed by an atom or molecule due to loss or gain of electrons.
Oxidizing Agent (OA) – Those chemical species that undergo reduction (R-OA)
Reducing Agent (RA) – Those chemical species that undergo oxidation (O-RA)
Spontaneous Reaction - The reaction that takes place naturally. Eg-Melting of ice cubes.
Non-Spontaneous Reaction – The reaction that takes place with the help of an external force. For Example: Burning of LPG needs initiation.
Cell Potential - The potential that a cell has, to do some work on its surrounding by the help of electric current flowing through the connected wire.
Fig. Example of spontaneous reaction.
Electrochemical Cells
It is of 2 types.
Parts of Galvanic Cell
Note: Daniel cell is a type of this cell. It was invented by a British chemist John F Daniel in 1836. And in this cell ZINC and COPPER electrodes and electrolyte is used.
Working of Galvanic Cell
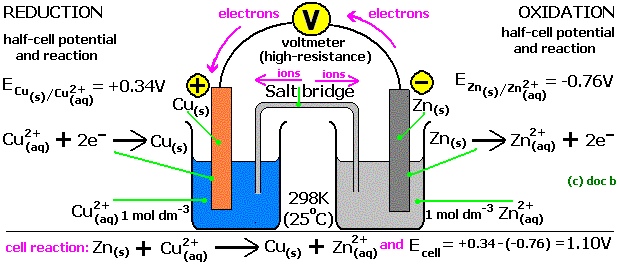
A unique property of a REDOX reaction is that, it can be carried out with the reactants present separately in space and just linked by an electrical connection i.e. chemical energy is converted into electrical energy. Let’s see a diagram of a galvanic cell which involves the reaction between cupric ions and zinc.
Fig: Galvanic cell of CuSO4 and ZnSO4
-
The setup consists of two beakers, one contains Cu2+ ions in it with copper rod as electrode, the second beaker contains Zn2+ solution and zinc rod as electrode. Since both of them are separated therefore in order to build connection between the two solutions, an inverted U tube is used which is known as Salt bridge. These contain agar-agar gel with electrolytic solution of KCl or NH4NO3.
-
The flow or leak of the solution from the salt bridge is avoided by plugging the ends of the tube with cotton or glass wool or even by capping with a porous material.
-
When the reaction starts the ammeter connected to the two electrode through wire shows deflection which confirms that a chemical reaction is occurring in the beakers and something charged is flowing.
-
Zinc electrode starts giving out Zn2+ ions in the electrolytic solution making it small with time, on the other hand the copper electrode increase in its size due to deposition of neutral copper atom on it. This will make the electrolytic solution of Zinc beaker more concentrated with cations and the other beaker lacks cations.
-
The ammeter deflection indicates that the electrons are moving from zinc rod to the copper rod. It’s a continuous process as long as reactants are enough, salt bridge is present and the electrical connection is firm.
-
Let’s see what happens inside on microscopic level, the zinc rod gives out electrons which come out of it and starts travelling through external circuit, this produces Zn2+ ions which have higher affinity towards solution medium than solid rod. Thus Zn2+ ions come out in beaker which leads to reduction in the size of zinc rod.
Fig: Oxidation of zinc rod
-
We even observe that electrons flow towards copper rod, and comes inside electrolytic solution, there it will neutralize the Cu2+ ions to metallic Cu atom which has high affinity towards solid Cu rod leading that they all gets deposited there on it, which increases its size.
Fig: Reduction of copper rod
-
Since Zinc beaker side loses electrons therefore it is called Oxidation Half-Cell, and copper beaker side gains electrons it is known as Reduction Half-Cell.
Fig: Redox reaction
The EO cell for this reaction is:
Fig: Eo cell equation.
Purpose of the Salt Bridge
Finally, we must understand the purpose and use of salt bridge. During reaction we have seen that Zinc ions are produced by losing electrons and this Zinc ions comes out in the solution, due to this the net positive charge of the zinc rod beaker increases.
On the same time, the overall negative charge on copper side beaker increases because Cu atom gets deposited on copper rod.
The salt bridge helps to prevent the net accumulation of positive and negative charges on both the sides. Doing so the negative ions from the salt bridge enter Zinc beaker side to reduce the net positive charge. The positive ions from the salt bridge enters copper side beaker to decrease net negative charge there.
If this was not done then due to accumulation of net positive and negative charges on both the sides the redox reaction will come to an end.
Thus we can tell that although salt bridge do not participate in the reaction directly but it help to maintain continuity of reaction.
Significance of Salt Bridge
 The following are the functions of the salt bridge:
The following are the functions of the salt bridge:
-
It helps to complete the connection of both half cells.
-
It prevents diffusion of the solutions in both the half cells.
-
It helps to build electrical neutrality.
-
It avoids liquid-liquid junction potential. (The potential difference arising when two liquids are in contact to each other.)
Note:
-
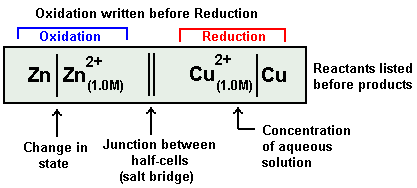
Two parallel vertical lines in a cell reaction indicate the salt bridge.
Zn|Zn2+||Cu2+|Cu
-
Salt bridge can be replaced by a porous partition which allows the migration of ions without allowing the solution to intermix.
Fig: Note on salt bridge.
IUPAC Cell Representation
Fig: Detailed diagram of Galvanic cell.
The Galvanic Cell mentioned above is represented in a short IUPAC cell notation as follows:
Fig: Galvanic cell notation / representation
It is important to note that:
It has total 3 parts:
-
Always anode electrode is written first on Left hand side. In above example it is Zn.
-
After solid anode its electrolyte is written next to it along with its concentration terms. In above example it is Zn2+ ion, its concentration term is written in bracket as subscript.
-
A straight slash is inserted between the electrode and its electrolyte. It represents a surface barrier between the electrode and electrolyte as the both exist in different state.
-
Salt bridge is represented as double vertical slash.
-
Reduction Side
-
Now electrolyte of cathode half-cell is written with its concentration term in bracket in subscript. In above example it is Cu2+ ion(1.0M)
-
A vertical slash is written after it,
-
Finally after it we write the cathode electrode of the cathode half-cell.
-
If gas is there, it is indicated after the electrode if it is on anode side and before the electrode in case of cathode. Example: (Pt, H2/H+ or H+|H2, Pt.)
Types of Voltaic Cells
Type |
Uses |
Advantages |
Disadvantages |
Images |
|
Mercury Cell (1.2 V) |
Watches Cameras |
Long lasting Provide fixed voltage |
Cannot to recharged |
|
|
Lead acid battery (12V) |
Car Truck |
Provide fixed voltage Rechargeable |
Spoiled fast. Heavy and expensive. |
|
|
Ni-Cd cell (1.25 – 1.35V) |
Mobile Emergency light |
Long lasting. Rechargeable. |
Expensive. |
|
|
Dry Cells (1.5 V) |
Radio Torch |
Provide fixed current Easy to carry. |
Non rechargeable. Short life |
|
|
Alkaline cell (1.5 V) |
Torch |
Long lasting Provide fixed current. |
Non rechargeable. Expensive |
Application of Galvanic Cells
Galvanic Cells are the basis of batteries that help our modern society to work smoothly. The current runs through the circuit to supply power and life to our devices. These cells are used in many rechargeable devices. These cells are now also used in solar power up gradation which is used in electric cars. These are used in:
-
Solar cells
-
Automobiles like in tesla cars.
-
Antennas used to receive and emit electromagnetic radiation
-
Used in small electric motors.
 Advantages of Galvanic Cells
Advantages of Galvanic Cells
-
They last for longer time.
-
It’s easily available
-
Provide steady flow of current and provide less fluctuations
Disadvantages of Galvanic Cells
-
Some cannot be recharged.
-
Some undergo rusting.
-
Some are too expensive.
-
Some need extra care else will be spoiled easily.
-
Some are bulky and heavy.
 |
 |
| Fig: Rusting seen in batteries. | Fig: Corrosion seen in batteries. |
Watch this Video for more reference
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free