Electrolytic Cell and Electrolysis
Table of Content |
|
|
Electrochemical Cells
The cell that can convert electric energy using a chemical process and vice versa is known as Electrochemical Cell. Best example of this is the battery we use in remote or wall clock which convert stored chemical energy to electrical energy.
This cells work on oxidation-reduction reaction or redox reaction concept.
| Oxidation | Reduction |
|
Addition of oxygen(s) |
Addition of hydrogen(s) |
|
Removal of hydrogen(s) |
Loss of oxygen(s) |
|
Loss of electron(s) |
Gain of electron(s) |
|
Increase in oxidation number(s) |
Decrease in oxidation number(s) |
|
Result in formation of many C-O bonds. |
Result in formation of many C-H bonds. |
|
The product formed will be having higher potential energy. |
Types of Electrochemical Cells
-
Electrolytic Cells
-
Galvanic Cells
Electrolytic Cells
Electrolytic Cells: In these cells electrical energy is used to carry out a non-spontaneous reaction. In nut shell, we can say that in galvanic cells, chemical energy is transformed into electrical energy, while in electrolytic cell electrical energy is converted into chemical energy.
Galvanic Cells
Galvanic Cells (also known as Voltaic Cell): It’s an instrument in which a redox reaction is used to convert chemical energy into electrical energy. The reaction used for generation of electricity takes place in two different cells. Each cell comprises of a suitable electrolytic solution and a metallic rod knows as Electrode (generally same type of electrode and electrolyte).The cells comprising of the electrode and the electrolytic solution are called Half-Cells. The moment two cells are connected via salt bridge and electrodes through a wire linked with galvanometer the electricity begin to flow. It’s a simple form of voltaic cell name after its discoverer an Italian scientist Alessandro Volta.
Note: Voltaic Cell is a type of this cell. It was invented by a British chemist John F Daniel in 1836. And in this cell ZINC and COPPER electrodes and electrolyte is used.
Electrolytic Cell Vs Galvanic Cell
Electrolytic Cell
-
In this cell a setup is made such as electricity is conducted through a electrolytic solution or its molten form or fused form which generates free ions, that’s why it is said rightly that electrical energy is converted to chemical energy in electrolytic cells.
-
The working principle of conduction by electrolytic cell is illustrated by a cell in which electrolysis of molten NaCl is done between inert or Nobel electrodes which do not react with it.
Fig: Redox cell
-
To get a proper setup of electrolytic cell, so that it helps to pass the current through aqueous solution of electrolyte that is, NaCl, two metallic rods are in it which is in turn is linked with the ends of a power source.
-
These two metallic rods are called electrodes, the rod through which current enters in the aqueous electrolytic solution is anode, its positive end of the setup and the rod through which current leaves the aqueous electrolytic solution is cathode, its negative end of the setup.
-
This entire setup excluding the external power source or battery is called Cell. The electrons flowing out through the negative electrode of the cell is received by negative terminal of battery.
-
These flowing electrons are used up in the reduction reaction which takes place at cathode.
-
The number of electrons accepted at the negative terminal of electrode is reverted back to the positive terminal of the power source via positive electrode of the cell, where electrons are coming out due to oxidation.
-
Inside the setup the current is carried out by the movements of the free ions generated during the electron flow in and out of the cell; the furnished cations moves towards cathode (negative electrode) and anions towards anode (positive electrode)
-
This free motion of both types of ions gives rise to electrolytic conduction.
Just Think
-
CASE-01 Multi cation presence in cell
-
Standard Reduction Potentials at 25° C are given below for few common reactions
-
CASE-02: For Multi anions presence in cell
-
Importance of Salt Bridge
CASE-01 Multi cation presence in cell
Let’s see a condition which involves more than one type of cations in it. The capability of a cation to move towards the cathode, where it will gain electron and gets reduced depends on the factors like its mass, size, charge on it etc.
Quoting this we can tell that it is difficult to predict, quantitatively, the correct order of reduction of cations. Because one factor may accelerate it and other may suppress.
The best possible way to predict this is by giving a quantitative value or number on the overall effect of all the factors involved in the reduction ability of a cation.
This quantitative number or value is called SRP-Standard reduction potential. A cation with higher SRP would get reduced preferentially faster than the cation with lower value of SRP.
Standard Reduction Potentials at 25° C are given below for few common reactions
|
Reducation half reaction |
E°, V |
Reducation half reaction |
E°, V |
|
F2 + 2e– → 2F– |
2.87 |
AgCl + e– → Ag + Cl– |
0.222 |
|
S2O82-+2e- → 2SO42- |
2.0 |
PdI42- + 2e– → Pd + 4I– |
0.18 |
|
Co3+ + e– → Co+2 |
1.82 |
Cu2+ + e– → Cu+ |
0.15 |
|
H2O2 + 2H+ + 2e– → 2H2O |
1.77 |
Sn4+ + 2e– → Sn2+ |
0.13 |
|
MnO4- + 4H+ + 3e– → MnO2 + 2H2O |
1.70 |
Ag(S2O3)23-+e– → Ag+ 2S2O32- |
0.017 |
|
PbO2 + 4H+ + SO42+ 2e– → PbSO4 + 2H2O |
1.70 |
2H+ + 2e– → H2 |
0.000 |
|
Ce4+ + e– → Ce3+ |
1.70 |
Pb2+ + 2e– → Pb |
– 0.126 |
|
MnO4- + 8H+ + 5e– → Mn+2 + 4H2O |
1.51 |
Sn2+ + 2e– → Sn |
– 0.14 |
|
Au3+ + 3e– → Au |
1.50 |
2CuO + H2O + 2e– → Cu2O + 2OH– |
– 0.15 |
|
Cl2 + 2e– → 2Cl– |
1.36 |
AgI + e– → Ag + I– |
– 0.151 |
|
Cr2O72- + 14H+ + 6e– → 2Cr+3 + 7H2O |
1.33 |
CuI + e– → Cu + I– |
– 0.17 |
|
Ti3+ + 2e– → Ti+ |
1.26 |
Ni2+ + 2e– → Ni |
– 0.25 |
|
MnO2 + 4H+ + 2e– → Mn2+ + 2H2O |
1.23 |
Co2+ + 2e– → Co |
– 0.28 |
|
O2 + 4H+ + 4e– → 2H2O |
1.229 |
PbSO4 + 2e– → Pb + SO42- |
– 0.31 |
|
2IO3- + 12H+ + 10e– → I2 + 6H2O |
1.20 |
Ti+ + e– → Ti |
– 0.336 |
|
Br2 + 2e– → 2Br– |
1.09 |
Cu2O + H2O + 2e– → 2Cu + 2OH– |
– 0.34 |
|
AuCl4- + 3e– → Au + 4Cl– |
1.00 |
Cd2+ + 2e– → Cd |
– 0.403 |
|
OCl– + H2O + 2e– → Cl– + 2OH– |
0.94 |
Fe2+ + 2e– → Fe |
– 0.44 |
|
Pd2+ + 2e– → Pd |
0.92 |
Cr3+ + 3e– → Cr |
– 0.74 |
|
2Hg2+ + 2e– → Hg22+ |
0.92 |
Zn2+ + 2e– → Zn |
– 0.7628 |
|
Cu2+ + I– + e– → CuI |
0.85 |
2H2O + 2e– → H2 + 2OH– |
– 0.828 |
|
Ag+ + e– → Ag |
0.799 |
Mn2+ + 2e– → Mn |
– 1.18 |
|
Fe3+ + e– → Fe2+ |
0.771 |
H2 + 2e– → 2H– |
– 2.25 |
|
O2 + 2H+ + 2e– → H2O2 |
0.69 |
Mg2+ + 2e– → Mg |
– 2.37 |
|
Cu2+ + Cl– + e– → CuCl |
0.566 |
Ce3+ + 3e– → Ce |
– 2.48 |
|
I2 + 2e–- → 2I– |
0.535 |
Na+ + e– → Na |
– 2.713 |
|
Cu+ + e– → Cu |
0.52 |
Ca2+ + 2e– → Ca |
– 2.87 |
|
Cu2+ + 2e– → Cu |
0.34 |
K+ + e– → K |
– 2.93 |
|
0.270 |
Li+ + e– → Li |
–3.03 |
|
|
Hg2Cl2 + 2e– → 2Hg + 2Cl– (satd KCl) |
0.244 |
|
|
|
Ge2+ + 2e– → Ge |
0.23 |
|
|
CASE-02: For Multi anions presence in cell
The reverse of SRP is called SOP-Standard oxidation potential.
In the electrolysis of aqueous solution of NaCl, H+ ion would be reduced to H2 gas by gain of electrons. (This H+ ion comes from water present as aqueous) at the cathode, on the other hand Cl- ions would be oxidized to Cl2 gas at the anode.
The above stated concepts are although used in solving problems, it is not valid always. Because the ability of a cation to get reduced or an anion to get oxidized are also dependent on their concentrations.
This tells that it is realistic to reduce a cation in preference to another cation although the SRP of the former cation is less than that of latter, simply by adjusting its concentration.
A unique property of an REDOX reaction is that , it can be carried out with the reactants present separately in space and just linked by an electrical connection that is, chemical energy is converted into electrical energy.
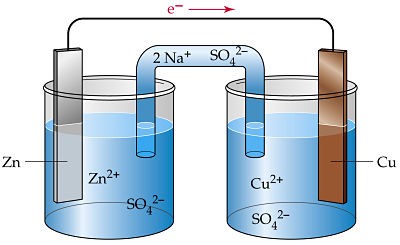
Let’s see a galvanic cell below in which reaction between cupric ions and metallic zinc is seen:
Fig: Redox Reaction Example
-
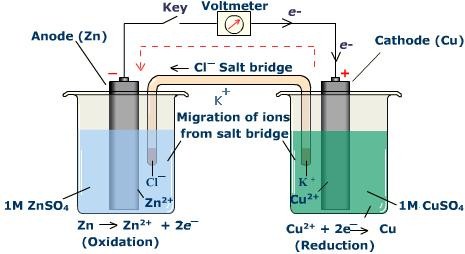
The setup consist of two beakers, one contains Cu2+ ions in it with copper rod as electrode, the second beaker contain Zn2+ solution and zinc rod as electrode. Since both of them are separated therefore in order to build connection between the two solutions an inverted U tube is used which is known as Salt Bridge. These contain agar-agar gel with electrolytic solution of KCl or NH4NO3.
-
The flow or leak of the solution from the salt bridge is avoided by plugging the ends of the tube with cotton or glass wool or even by capping with a porous material.
-
When the reaction starts the ammeter connected to the two electrode through wire shows deflection which confirms that a chemical reaction is occurring in the beakers and something charged is flowing.
-
Zinc electrode starts giving out Zn2+ ions in the electrolytic solution making it small with time, on the other hand the copper electrode increase in its size due to deposition of neutral copper atom on it. This will make the electrolytic solution of Zinc beaker more concentrated with cations and the other beaker lacks cations.
-
The ammeter deflection indicates that the electrons are moving from zinc rod to the copper rod. It’s a continuous process as long as reactants are enough, salt bridge is present and the electrical connection is firm.
-
Let’s see what happens inside on microscopic level, the zinc rod gives out electrons which come out of it and starts travelling through external circuit, this produces Zn2+ ions which have higher affinity towards solution medium than solid rod. Thus Zn2+ ions come out in beaker which leads to decrease in size of Zn rod.
|
Zn (s) → Zn2+ + 2e– ( zinc rod decrease in size) |
-
We even observe that electrons flow towards copper rod, and comes inside electrolytic solution, there it will neutralize the Cu2+ ions to metallic Cu atom which has high affinity towards solid Cu rod leading that they all gets deposited there on it, which increases its size.
|
2e– + Cu2+ (aq) → Cu (s) (copper rod increase in size) |
-
Since Zinc beaker side loses electrons therefore it is called Oxidation Half-Cell, and copper beaker side gains electrons it is known as Reduction Half-Cell.
Finally, we must understand the purpose and use of salt bridge. During reaction we have seen that Zinc ions are produced by losing electrons and this Zinc ions comes out in the solution, due to this the net positive charge of the zinc rod beaker increases.
On the same time the overall negative charge on copper side beaker increases because Cu atom gets deposited on copper rod.
The salt bridge helps to prevent the net accumulation of positive and negative charges on both the sides. Doing so the negative ions from the salt bridge enter Zinc beaker side to reduce the net positive charge. The positive ions from the salt bridge enters copper side beaker to decrease net negative charge there.
If this was not done then due to accumulation of net positive and negative charges on both the sides the redox reaction will come to an end.
Thus we can tell that although salt bridge do not participate in the reaction directly but it help to maintain continuity of reaction.
Importance of Salt Bridge
The following are the functions of the salt bridge:
-
It helps to complete the connection of both half cells.
-
It prevents diffusion of the solutions in both the half cells.
-
It helps to build electrical neutrality.
-
It avoids liquid-liquid junction potential. (The potential difference arising when two liquids are in contact to each other.)
Note:
-
Two parallel vertical lines in a cell reaction indicate the salt bridge.
Zn|Zn2+||Cu2+|Cu
Salt bridge can be replaced by a porous partition which allows the migration of ions without allowing the solution to intermix.
Fig: Salt Bridge Note.
IUPAC Cell Representation
Fig: Cell Representation
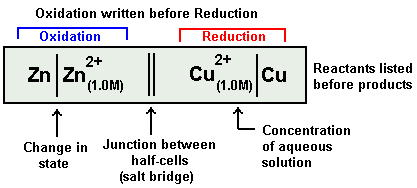
The galvanic cell explained above is represented in a short IUPAC cell notation as follows:
Fig: IUPAC cell notation
Tricks to write cell notation:
It has total 3 parts
-
Oxidation Side
-
Salt Bridge
-
Reduction Side
Oxidation Side
-
Always anode electrode is written first on Left hand side. In above example it is Zn.
-
After solid anode its electrolyte is written next to it along with its concentration terms. In above example it is Zn2+ ion, its concentration term is written in bracket as subscript.
-
A straight slash is inserted between the electrode and its electrolyte. It represents a surface barrier between the electrode and electrolyte as the both exist in different state.
Salt Bridge
Salt bridge is represented as double vertical slash.
Reduction Side
-
Now electrolyte of cathode half-cell is written with its concentration term in bracket in subscript. In above example it is Cu2+ ion(1.0M)
-
A vertical slash is written after it,
-
Finally after it we write the cathode electrode of the cathode half-cell.
-
If gas is there, it is indicated after the electrode if it is on anode side and before the electrode in case of cathode. Example: :(Pt, H2/H+ or H+|H2, Pt.)
Make a note for Electrode difference
What is Electrolysis?
The word electro means electricity and lysis means kill.
It is the phenomena of separating a compound into its participating/constituent elements by passing an electric current through its molten condition or aqueous solution.
Preferential Discharge Theory
This theory explains that if an electrolyte contains more than two ions and electrolysis is performed, it is seen that all the ions are not discharged at the electrode at the same time but certain ions are liberated at the electrode in preference to others.
According to this theory if all the ions are attracted towards a particular electrode, then the one which needs least energy to gets discharged is discharged first than others.
The potential at which the ions are discharged is called deposition or discharge potential.
Discharge potential value is different for different ions. Like discharge potential of Na+ ions are more than that of H+ ions when inert electrodes like platinum or other metal electrodes are used as cathode. Just like this discharge potential of OH-is greater than that of Cl- ions. The above explanation can be explained below:
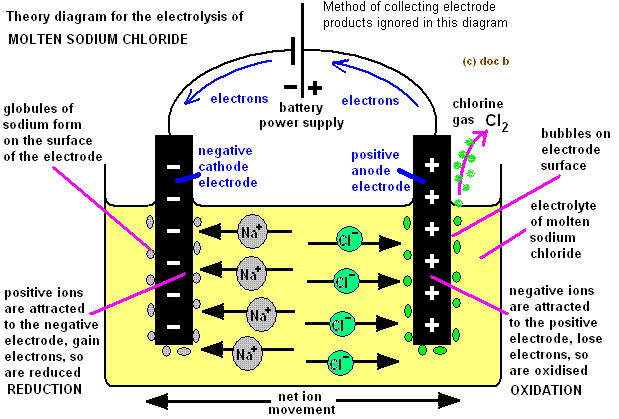
1. Electrolysis of NaCl Solution
The aqueous solution of NaCl contains ion like Na+, Cl- , H+ and OH- ions which comes from NaCl and ionization of water. Since water is a weak electrolyte its ion population is less. During reaction when potential difference is reached between the two electrodes Na+ and H+ ions move towards negative electrode that is cathode and Cl- and OH- ions move towards positive electrode or anode.
At cathode between H+ and Na+ ions H+ ions are discharged, on the same ground Cl- ions are discharged in preference to OH- ions.
NaCl ⇌ Na+ + Cl-
H2O ⇌ H+ + OH-
| At cathode | At Anode |
| H+ + e- → H | Cl- → Cl + e- |
| 2H→ H2 | 2Cl → Cl2 |
Therefore, Na+ and OH- ions remains undisturbed in the aqueous solution and when the solution is evaporated it yields crystals of NaOH.
Fig: Molten NaCl electrolysis
2. Electrolysis of CuSO4 solution using platinum electrodes
CuSO4 ⇌ Cu2+ + SO42-
H2O ⇌ H+ + OH-
| At cathode | At Anode |
| Cu2+ + 2e- → Cu | 2OH- → H2O + O + 2e- |
| O + O → O2 |
Between Cu2+ ions and H+ ions Cu2+ ions is discharged at cathode, because Cu2+ ions have lower discharge potential, between OH- and SO42- , OH- ions discharged at anode due to its lower discharge potential. Finally copper is deposited at cathode and oxygen gas is released at anode.
3. Electrolysis of Na2SO4 solution using inert electrodes
Na2SO4 ⇌ 2Na+ + SO42-
H2O ⇌ H+ + OH-
| At cathode | At Anode |
| H+ + e- → H | OH- → H2O + O + 2e- |
| 2H→ H2 | O + O → O2 |
Between H+ and Na+ ions H+ ions are discharged at cathode as H+ ions have lower discharge potential than Na+ ions. Between SO42- and OH-, OH- ions are discharged at anode as these have lower discharge potential. Thus, hydrogen gas is released at cathode and oxygen gas at anode, that is, the net reaction describes the electrolysis of water. The ions of Na2SO4 conduct the current through the solution and take no part in the overall chemical reaction.
The decreasing order of discharge potential or the increasing order of deposition of some of the ions is given below:
For cations: K+> Na+> Ca2+> Mg2+> Al3+>Zn2+> H+> Cu2+> Hg2+> Ag+
For anions: SO42-> NO3-> OH->Cl-> Br-> I-
4. Electrolysis of copper sulphate solution using copper electrodes
CuSO4 ⇌ Cu2+ + SO42-
Copper is deposited at cathode,
Cu2+ + 2e- → Cu
At anode, the copper of the electrode is oxidised to Cu2+ ions or ions solution dissolve equivalent amount of copper of the anode.
Cu → Cu2+ + 2e-
Thus, during electrolysis, copper is transferred from anode to cathode.
5. Electrolysis of silver nitrate solution using silver electrodes
AgNO3 ⇌ Ag+ + NO3-
Silver is deposited at cathode,
Ag+ + e- → Ag
At anode, the silver of the electrode is oxidised to Ag+ ions which go into the solution or ions dissolve equivalent amount of silver of the electrode.
Ag → Ag+ + e-
Ag + NO3- → AgNO3 + e-
Some More Examples of Electrolysis
| Electrolyte | Electrode | Cathodic Reaction | Anodic Reaction |
|
Aqueous acidified CuCl2 solution |
Pt | Cu2+ + 2e-→ Cu | 2Cl- → Cl2 + 2e- |
|
Molten PbBr2 |
Pt | Pb2+ + 2e- → Pb | 2Br- --> Br2 + 2e- |
| Hg | 2Na+ + 2e- → 2Na | 2Cl- → Cl2 + 2e- | |
|
Silver nitrate solution |
Pt | Ag+ + e- --> Ag | 2OH- → 1/2 O2 + H2O + 2e- |
|
Sodium nitrate solution |
Pt | 2H+ + 2e- → H2 | 2OH- → 1/2 O2 + H2O + 2e- |
Frequently Asked Questions (FAQs)
Q1. Electrolytic cell converts.
a. electrical energy into chemical energy.
b. chemical energy into electrons.
c. chemical energy into electrical energy.
d. electrical energy into ions.
Sol. a
Q2. Which of the following objects is not part of electrochemical cell
a. Anode
b. Battery
c. Cathode
d. Electrolyte
Sol. b
Q3. Which of the following statements are incorrect regarding salt bridge?
a. It connects the solutions of two half-cells and completes the cell circuit.
b. It prevents transference or diffusion of the solutions from one half-cell to the other.
c. It keeps the solutions in two half-cells electrically neutral.
d. It causes liquid-liquid junction-potential
Sol. d
Q4. Which of the following gas is produced at anode during electrolysis of sodium chloride?
a. Oxygen
b. Hydrogen
c. Chlorine
d. Nitrogen
Sol. c
Q5. Which of the following ions has maximum discharge potential?
a. K+
b. Na+
c. Ca2+
d. Mg2+
Sol. a
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More