Emulsions
Table of Content |
 The liquid-liquid colloidal dispersions are called emulsions. An emulsion may be defined as follows:
The liquid-liquid colloidal dispersions are called emulsions. An emulsion may be defined as follows:
The colloidal dispersion of two immiscible liquids in which one liquid acts as the dispersion medium and the other as dispersed phase is called an emulsion.
Types of Emulsion
Depending upon the nature of dispersed phase, emulsions can be classified into following two types.
-
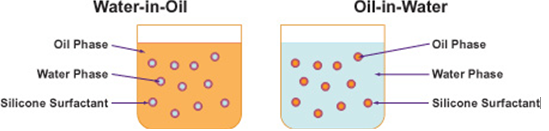
Oil-in-water (O/W) type emulsions: In oil-in-water emulsions, an oil acts as the dispersed phase while water acts as the dispersion medium. The most common example of oil in water type emulsion is milk which consists of liquid fat globules dispersed in water.
-
Water-in-oil (W/O) type emulsions: In water-in-oil type emulsions, water acts as the dispersed phase, whereas oil acts as the dispersion medium. This type of emulsions is also referred to as oil emulsions. Cod liver oil emulsion is a typical example of this type of emulsions in which water is dispersed in cod liver oil. The two types of emulsions are diagrammatically shown in figure below.
Preparation of Emulsions
Emulsions are usually prepared by vigorously mixing the two liquids by using either a high speed mixing machine or by using ultrasonic vibrators. The process is known as emulsification. Since the two liquids used for the preparation of an emulsion are completely immiscible, a stabilizing substance, known as emulsifying agent or emulsifier is required to stabilize the resulting emulsion.
The emulsifier is added along with the component liquids. In the absence of emulsifying agent, the dispersed phase particles of colloidal size combine together resulting in the breaking up of emulsion into two separate layers. Some of the important emulsifying agents are soaps, detergents, proteins, gums and agar. Among these, soaps and detergents are most commonly used emulsifiers.
Role of Emulsifier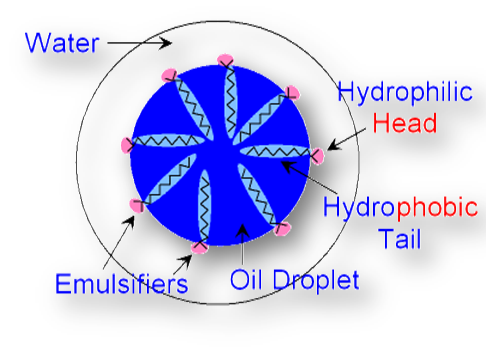
The emulsifiers form a protective film around the oil droplets dispersed in water. This prevents them to come closer and to coalesce, i.e. to combine together. Thus, the emulsion gets stabilized. For example, let us consider the role of soap which acts as an emulsifier for an oil-in-water emulsion.
When soap is added to an o/w emulsion, the soap molecule (RCOO-Na+) arrange themselves in such a way that the polar end groups dip in water whereas the hydrocarbon chains dip in oil droplet as shown in the figure below. Thus soap molecules get concentrated over the surface of the oil droplet and form a protective film. This decreases the interfacial between oil and water and the emulsion gets stabilized.
Identification of Emulsion type
The type to which an emulsion belongs can be known as any of the following tests.
-
Dilution test: This test is carried out by adding a few drops of water to the given emulsion. If the added water mixes freely with the emulsion, the emulsion is of oil-in-water (o/w) type. In case the added water does not mix up with the emulsion, the given emulsion is of water-in-oil (w/o) type. The experiment can also be carried out by adding a few drops of oil instead of water. If the added oil gets mixed up, the emulsion is of water-in-oil type.
-
Conductivity test: This test involves the addition of a small amount of electrolyte to the emulsion under the examination followed by the measurement of its conductance. If the conductance increases, the emulsion is of oil-in-water type. In case, there is no appreciable change in the conductance, the emulsion is of water-in-oil type.

-
Dye test: In this test, a small amount of an oil-soluble dye is added to the emulsion. If the emulsion becomes coloured, it is of water-in-oil type. If no change in colour is observed, the emulsion is of oil-in-water type.
Properties of Emulsions
The size of the dispersed droplets in emulsions may be somewhat larger as compared to the size of dispersed particles in sols. Still, emulsions are colloidal systems and exhibit all the properties exhibited by colloidal solutions e.g. Brownian movement, Tyndall effect, electrophoresis, coagulation etc.
Emulsions can be broken into their constituent liquids by physical methods such as heating, freezing, centrifuging etc. or by destroying the emulsifying agent by a suitable chemical method. The process of breaking an emulsion to yield the constituent liquids is called demulsification.
Applications of Emulsions
Emulsions are very useful systems and find a number of applications. Some important applications of emulsions are given below.
-
A large number of pharmaceuticals are prepared in the form of lotions, creams and ointments which are emulsions of oil-in-water or water-in-oil type and are easily absorbed by the body. Similarly, many cosmetics are also sold as emulsions. Several oily drugs are also prepared as emulsions to facilitate their absorption.
-
The concentration of sulphide one by froth floatation process involves the treatment of finely pulverized one with an oil emulsion. When air is bubbled in the mixture, the ore particles present in the emulsion are carried to the surface in the form of foam and may be collected.
-
Emulsifying properties of soaps and detergents are used in washing clothes, crockery etc. Soaps and detergents emulsify the greasy dirt and carry it away in the water used for washing.
-
Digestion of fats in the intestine is facilitated by emulsification. A small amount of fat reacts with the alkaline solution of the intestine to form sodium soap. The sodium soap thus formed emulsifies the rest of the fat. This helps digestive enzymes to carry out their functions in a better way and the fats get digested easily.
-
In spite of several applications useful in our daily life, formation of emulsions sometimes creates difficulties also. For example, some all wells yield emulsified petroleum instead of petroleum alone. However, such emulsions can be demulsified to yield the constituent liquids by physical methods mentioned earlier. ?
Gels |
|
A gel is a jelly like colloidal system in which a liquid is dispersed in a solid medium. Gel may be classified into two types:
|

Question 1: Emulsions is a
a. solid - solid system.
b. liquid -solid system.
c. liquid- liquid system.
d. gas -liquid system.
Question 2: Emulsification is the process of
a. purification of emulsions.
b. preparation of emulsions.
c. evaporation of emulsions.
d. precipitation of emulsions.
Question 3:
In the cleaning of cloths, soap acts as ___ for oil in water system?
a. emulsifier
b. dispersion medium
c. dispersed phase
d. coagulating agent
Question 4: Which of the following tests is not used for identification of emulsion type ?
a. Dilution test
b. Conductivity test
c. Dye test

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
c |
b |
a |
d |
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Click here access the syllabus of chemistry for IIT JEE
-
Click here for classification of colloids
To read more, Buy study materials of Surface Chemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More