Significant Figures and Scientific Notation
Precision and Accuracy
Precision refers to the closeness of various measurements for the same quantity while accuracy refers to the agreement of a particular value to the true value of the result.
For example, let the true value for a result be 2.00 g. A student ‘x’ takes two measurements and reports the result as 1.95 g and 1.93 g. These values are precise but not accurate. Another student ‘Y’ repeats the experiment and reports the result as 1.94 g and 2.05 g. These values are neither precise nor accurate.
A third student ‘z’ repeats the measurement and reports the result as 2.01g and 1.99 g. These values are both precise and accurate.

Significant Figures
Significant figures are total number of digits in any number including the last digit whose value is uncertain.
Rules for Calculating Number of the Significant Figures
-
All non-zero digits are significant. For example, 866 has three significant figures and 0.866 also has three significant figures..
-
Zeros to the left of the first non-zero digit in the number are not significant. For example, 0.008 has only one significant figure and 0.0085 has two significant figures.
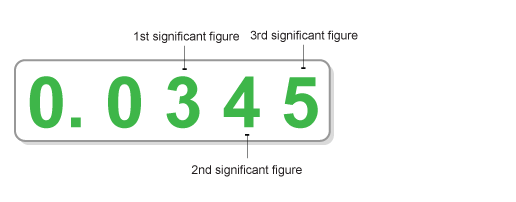
-
Zeros between non-zero digits are significant. For example, 3.05 has three significant figures.
-
Zeros to the right of the decimal point are significant. For example, 6.00, 0.60 and 0.6000 have 3, 2 and 4 significant figures.
-
If a number ends with zeros that does not have decimal, the zeros are not significant. For example: 3800 has two significant figures.

Refer to the following video for scientific figures
Scientific Notation:
Scientific notation is the representation of numbers in N x 10n form.
Where N = a number with a single non-zero digit to the left of the decimal point.
n = some integer.
For example, 3800 can be expressed in scientific notation in the following forms depending upon whether it has two three or four significant figures:
Scientific Notation of 3800 |
|
|
3.8 x 103 |
Two Significant Figures |
|
3.80 x 103 |
Three Significant Figures |
|
3.8000 x 103 |
Four Significant Figures |
|
0.38 x 104 |
Two Significant Figures |
|
0.038 x 104 |
Two Significant Figures |
-
Thus, we can write 531.598 as 5.31598 ×102 in scientific notation. Note that while writing it, the decimal had to be moved to the left by two places and same is the exponent (2) of 10 in the scientific notation.
-
Similarly, 0.00091 can be written as 9.1 × 10–4. Here the decimal has to be moved four places to the right and ( – 4) is the exponent in the scientific notation.
Calculation with Significant Figures:
Carrying out calculations with these numbers, the rule used is that the accuracy of the final result is limited to the least accurate measurement
Rounding Off
The rules employed for rounding off a number to the required number of significant digit are as follows
-
If the digit to be removed is more than 5, the digit to be retained is increased by one. for example, 2.486 If we have to remove 6, we have to round it to 2.49
-
If the digit to be removed is less than 5, the digit to be retained i.e. preceding number is not changed. For example, 5.334 if 4 is to be removed, then the result is rounded upto 5.33.
-
If the rightmost digit to be removed is 5, then the preceding number is not changed if it is an even number but it is increased by one if it is an odd number. For example, if 7.35 is to be rounded by removing 5, we have to increase 3 to 4 giving 7.4 as the result. However, if 7.25 is to be rounded off it is rounded off to 7.2.
Addition and Subtraction:
In addition and subtraction the final result should be reported to the same number of decimal places as the number with the minimum number of decimal places.

37.768 is rounded off in such a way that there is only one digit after the decimal.
For removing 6, 1 is added to 7 as 6 is greater than 5.
|
Example: 2.435 + 1.23 = 3.665 But the result would be reported only upto 2 decimal places so the answer would be 2.66. Example : 44.341- 1.4432 = 42.8978. But the result would be reported only upto 3 decimal places so the answer would be 42.898. |
Multiplication and Division:
In multiplication and division, the final result should be reported as having the same number of significant digits as the number with least number of significant digits.

|
Example: 3.9898 x 0.23 = 0.917654 But the result would be reported in the form with two significant figures so the answer would be 0.92. |


Question 1: How many significant figures are there in 1001?
a. 4
b. 2
c. 1
d. 3
Question 2: In the final answer of the expression

the number of significant figures would be
a. 1
b. 2
c. 3
d. 4
Question 3: Final result of the expression
10.23 + 11.234 - 22.1 – 11.2345
should be reported to the same number of decimal places as
a. 10.23
b. 11.234
c. 22.1
d. 11.2345
Question 4: Which of the following alternatives represents an incorrect scientific notation for the number 123.45 ?
a. 12345.00 x 10-2
b. 1.23 x 102
c. 0.0123 x 103
d. 12.34 x 10-1

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
c |
c |
d |
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Click here to know the syllabus of chemistry for IIT JEE
-
You can also have a look at matter and Its nature
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free