Gravimetric Analysis
Table of Content |
Gravimetric analysis is an analytical technique based on the measurement of mass of solid substances and/or volume of gaseous species.
The gravimetric analysis is broadly classified into three heads.
-
Mass – mass relationship
-
Volume - volume relationship
-
Mass- volume relationship
Mass- Mass Relationship
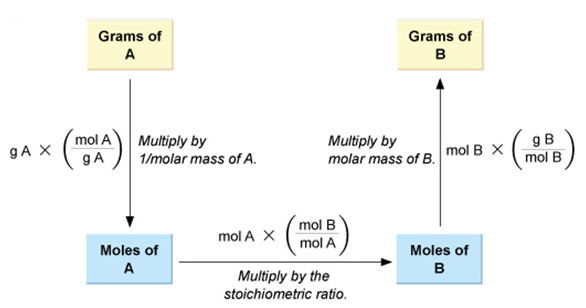
This relates the mass of a species (reactant or product) with the mass of another species (reactant or product) involved in a chemical reaction.
Let us consider a chemical reaction,
CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
Let the mass of CaCO3 taken be x g and we want to calculate the mass of CaO obtained by heating x g of CaCO3. Then the moles of CaCO3 taken would be ![]() (where M1 represents the molar mass of CaCO3).
(where M1 represents the molar mass of CaCO3).
According to the balanced reaction, the molar ratio of CaCO3 and CaO is 1 : 1, so same number of moles ii.e. ![]() , of CaO would be formed.
, of CaO would be formed.
Now for converting the moles of CaO into mass of CaO obtained, we need to multiply the moles of CaO with the molar mass of CaO. Let the molar mass of CaO be M2, so the mass of CaO obtained by heating x g of CaCO3 would be ![]() g.
g.
CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
We want to find out the volume of CO2 evolved at STP by heating x g of CaCO3. Then
Moles of CaCO3 = ![]()
Moles of CO2 evolved = ![]() (since molar ratio of CaCO3 and CO2 is 1 : 1)
(since molar ratio of CaCO3 and CO2 is 1 : 1)
Hence Volume of CO2 evolved at STP = ![]() L
L
But, if the volume of CO2 evolved is to be calculated at pressure P atm and temperature T K. Then,
Volume of CO2 evolved at pressure P and temperature T = ![]() ( Using PV = nRT)
( Using PV = nRT)
Volume-Volume Relationship
This relationship deals with the volume of a gaseous species (reactant or product) with the volume of another gaseous species (reactant or product) involved in a chemical reaction.
Let us consider the reaction, N2(g) + 3H2(g) → 2NH3(g).
We are given x L of N2 at pressure P atm and temperature TK and we want to know the volume of H2 required to react with it at the same pressure and temperature.
Moles of N2 = ![]()
Moles of H2 required = ![]() (since molar ratio of N2 & H2 is 1 :3)
(since molar ratio of N2 & H2 is 1 :3)
Hence Volume of H2 required at same pressure & temperature = ![]() = 3 x L.
= 3 x L.
This result could also have been obtained by knowing that for a gaseous relation, at the same pressure and temperature, the moles of gas is directly proportional to volume of the gas (V a n since P & T are constant) or molar ratio and volume ratio are same.
Moles of H2 required = ![]()
Hence Volume of H2 required at pressure P’ atm & temperature T’K.
= ![]()
Refer to the following video for problems on stoichiometry
Now let us see the usefulness of these concepts in solving problems.

Mass – Volume Relationship
This relationship deals with the volume of a gaseous species (reactant or product) with the mass of a solid species (reactant or product) involved in a chemical reaction.
Let’s take an example of combustion of Mg in air
2Mg + O2 → MgO
In this reaction, one can find out the volume of O2 required to react with given mass of Mg.
Let the mass of Mg taken is x g. So, the number of moles of Mg taken would be , where M is the molar weight of Mg.
From the balanced equation, we know that the moles of O2 required is half of the number of moles of Mg taken i.e.
Now we know that one mole of O2 gas at S.T.P =22.4L
So, mole of O2 =
L

Question 1: What is the molar ratio of reactant and one of the products in the following reaction?
CaCO3(s) ![]() CaO(s) + CO2(g)
CaO(s) + CO2(g)
a. 1:1
b. 2:1
c. 3:1
d. 1:2
Question 2: Volume of 1 mole O2 gas at S.T.P is
a. 22.4 L
b. 2.24 L
c.2.24 L
d. 24.22 L
Question 3: How many moles of H2O are required to react with 46 g Na ?
Na + H2O → NaOH + H2
a. 1
b. 2
c. 3
d. 4
Question 4: What is the mass of NH3 produced from 22.4 L N2 gas at STP
N2(g) + 3H2(g) → 2NH3(g) ?
a. 36 g
b. 26 g
c. 17 g

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
a |
b |
a |
Related Resources
-
Look here for past year papers of IIT JEE
-
Click here to refer syllabus of chemistry for IIT JEE
-
Have a look at stoichiometry
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More