Properties of Colloids
Table of Content |
|
|
General Physical Properties of Colloidal Solutions
Following are the important physical properties of colloidal solutions:
-
Heterogeneity: Colloidal solutions are heterogeneous in nature. These consist of two phases-dispersed phase and dispersion medium.
-
Visibility of dispersed particles: Although colloidal solutions are heterogeneous in nature, yet the dispersed particles present in them are not visible to the naked eye and they appear homogenous. This is because colloidal particles are too small to be visible to the naked eye.
-
Filterability: Due to very small size, the colloidal particles pass through an ordinary filter paper. However, they can be retained by animal membranes, cellophane membrane and ultrafilters.
-
Stability: Lyophilic sols in general and lyophobic sols in the absence of substantial concentrations of electrolytes are quite stable and the dispersed particles present in them do not settle down even on keeping. However, on standing for a long time, a few colloidal particles of comparatively larger size may get sedimented slowly.
-
Colour: The colour of a colloidal solution depends upon the size of colloidal particles present in it. Larger particles absorb the light of longer wavelength and therefore transmit light of shorter wavelength. For example, a silver so having particles of size 150nm appears violet, whereas that having particles of size 60nm appears orange yellow.
Refer to the following video for properties of colloidal solutions
Optical Properties of Colloids (Tyndall Effect) 
-
When an intense converging beam of light is passed through a colloidal solution kept in dark, the path of the beam gets illuminated with a bluish light. This phenomenon is called Tyndall effect and the illuminated path is known as Tyndall cone.
The phenomenon was first observed by Tyndall in 1869. -
The Tyndall effect is due to the scattering of light by colloidal particles. Since the dimensions of colloidal particles are comparable to the wavelength of ultraviolet and visible radiations, they scatter these and get illuminated.
-
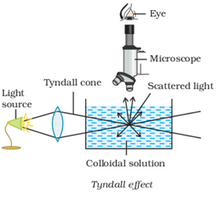 Tyndall observed that the zone of scattered light is much larger than the particle itself. This is why colloidal particles look like bright spots when viewed with a microscope at right angles to the beam of light as shown in figure. Thus, Tyndall effect may be defined as the scattering of light by colloidal particles present in a colloidal solution.
Tyndall observed that the zone of scattered light is much larger than the particle itself. This is why colloidal particles look like bright spots when viewed with a microscope at right angles to the beam of light as shown in figure. Thus, Tyndall effect may be defined as the scattering of light by colloidal particles present in a colloidal solution. -
Tyndall effect is not exhibited by true solutions. This is because the particles (ions or molecules) present in a true solution are too small to scatter light. Thus,
-
Tyndall effect can be used to distinguish a colloidal solution from a true solution. The phenomenon has also been used to devise an instrument known as ultra microscope. The instrument is used for the detection of the particles of colloidal dimensions. Tyndall effect also establishes the fact that colloidal systems are heterogeneous in nature.
 Mechanical Properties (Brownian Movement)
Mechanical Properties (Brownian Movement)
Colloidal particles present in a colloidal solution exhibit a very important property called Brownian movement. When a colloidal solution is viewed under an ultra microscope, the colloidal particles are seen continuously moving in a zigzag path. The property was discovered by a botanist Robert Brown in 1827, when he observed that pollen grains suspended in water exhibit random zigzag motion. After the name of the discoverer, the property was named as Brownian movement. It may be defined as follows.
The continuous zigzag movement of the colloidal particles in the dispersion medium in a colloidal solution is called Brownian movement.
Cause of Brownian Movement
Brownian movement is due to the unequal bombardments of the moving molecules of dispersion medium on colloidal particles. The moving molecules of the dispersion medium continuously attack on colloidal particles from all sides and impart momentum to them.
Since the chances of their collisions are unequal, the net driving force on a colloidal particle forces it to move a particular direction. As the particle moves in that direction, other molecules of the medium again collide with it and the particle changes its direction. The process continues. This results in a random zigzag movement of the colloidal particle.
The Brownian movement decreases with an increase in the size of colloidal particle. This is why suspensions do not exhibit this type of movement. Brownian movement plays an important role in imparting stability to a sol. This is because Brownian movement opposes the gravitational forces acting on colloidal particles and prevents them from getting settled down.
Electrical Properties of Colloidal Solutions
Some important electrical properties of colloidal solutions are as follows:
Presence of electrical charge on colloidal particles and stability of sols
One of the most important properties of colloidal solutions is that colloidal particles posses a definite type of electrical charge. In a particular colloidal solution, all the colloidal particles carry the same type of charge, while the dispersion medium has an equal but opposite charge. Thus, the charge on colloidal particles is balanced by that of the dispersion medium and the colloidal solution as a whole is electrically neutral. For example, in a ferric hydroxide sol, the colloidal ferric hydroxide particles are positively charged, while the dispersion medium carries an equal and opposite negative charge.
The stability of a colloidal solution is mainly due to the presence a particular type of charge on all the colloidal present in it. Due to the presence of similar and equal charges, the colloidal particles repel one another and are thus unable to combine together to form larger particles. This keeps them dispersed in the medium and the colloidal remains stable. This is why sol particles do not settle down even on standing for a long time.
Based on the nature of charge, the colloidal sols may be classified as positively charged and negatively charged sols. Some common examples of these sols are given below.
-
Positively charged sols: Metallic hydroxide sols e.g., Fe(OH)3, Al(OH)3, Cr(OH)3, etc., TiO2 sol, haemoglobin, sols of basic dyes such as methylene blue etc.
-
Negatively charged sols: Metal sols e.g., Au, Ag, Cu, Pt etc. sols, metal sulphide sols e.g., As2S3, CdS etc. sols; starch sol, sols of acid dyes such as Congo red etc.
Origin of Charge on Colloidal Particles 
There are several views regarding the origin of charge on colloidal particles. According to these views, colloidal particles acquire charge due to the following reasons.
-
Due to dissociation of the adsorbed molecular electrolytes: Colloidal particles have a strong tendency to adsorb reactant or product molecules. The molecules thus adsorbed on the surface of colloidal particles may undergo dissociation/ionization and may impart charge to them.
For example, during the preparation of sulphide sols (e.g., As2S3 sol), H2S molecules get adsorbed on colloidal particles. H2S molecules thus adsorbed undergo ionization and release H+ ions into the medium. Consequently, colloidal particles are left with negative charge. -
Due to the dissociation of molecules forming colloidal aggregates: The molecules responsible for the formation of aggregates of colloidal dimensions may themselves undergo dissociation/ionisation resulting in the development of charge on the colloidal particles formed by their aggregation.
For example, the soap molecules (RCOONa) dissociate to give RCOO- and Na+ ions. RCOO- ions aggregate together to form micelles which carry negative charge as explained earlier. -
Due to preferential adsorption of ions from solutions: The colloidal particles have a tendency to preferentially adsorb a particular type of ions from the solution. A colloidal particle usually adsorbs those ions which are in excess and are common to its own lattice.
This preferential adsorption of a particular type of ions imparts a particular type of charge to colloidal particles.
For example, when a ferric hydroxide sol is prepared by the hydrolysis of ferric chloride in warm water, the colloidal particles of Fe(OH)3 formed have a tendency to adsorb preferentially the Fe3+ ions present in the solution. This is because Fe3+ ions are common to the lattice of Fe(OH)3 particle. The Fe3+ ions thus adsorbed impart positive charge to the colloidal particles present in the sol.Fe(OH)3 + Fe3+ → Fe(OH)3 : Fe3+
(colloidal (ions common preferential adsorption of Fe3+ ions
particle) to the lattice of (colloidal particle acquires
colloidal particle) positive charge)Similarly, during the preparation of AgCl sol using excess of KCl solution, the Cl– ions are preferentially adsorbed and the colloidal particles acquire negative charge. However, if an excess of AgNO3 is used, Ag+ ions get preferentially adsorbed and the colloidal particles acquire positive charge.
Electrophoresis
Due to the presence of a particular type of electrical charge, the colloidal particles present in a colloidal dispersion move towards a particular electrode under the influence of an electric field.
The direction of movement of the colloidal particles is decided by the nature of charge present on them. If the colloidal particles carry positive charge, they move towards cathode when subjected to an electric field and vice versa. The phenomenon is called electrophoresis and may be defined as the movement of colloidal particles towards a particular electrode under the influence of an electric field.
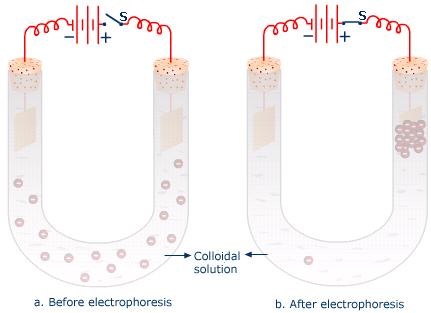 The phenomenon of electrophoresis clearly indicates that the colloidal particles carry a particular type of charge. The property can be used to find the nature of charge carried by colloidal particles in a colloidal dispersion.
The phenomenon of electrophoresis clearly indicates that the colloidal particles carry a particular type of charge. The property can be used to find the nature of charge carried by colloidal particles in a colloidal dispersion.
Electrophoresis is an important phenomenon and finds several applications in industry.
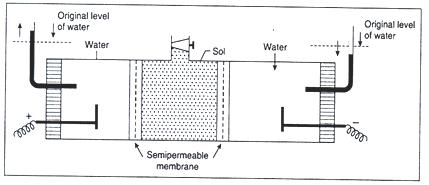
Electro-Osmosis
-
When the movement of colloidal particles under the influence of the applied electric field is checked with the help of a suitable membrane (semi permeable membrane), the dispersion medium moves in a direction opposite to the direction in which the colloidal particles would have otherwise moved. This phenomenon is called electro-osmosis and may be defined as the movement of dispersion medium under the influence of an electric field in the situation when the movement of colloidal particles is prevented with the help of a suitable membrane.
-
The colloidal solution is placed between two partitions made by semi permeable membranes.
-
The outer compartments consisting of platinum electrodes and side tubes are filled with water.
-
On passing electric current, water level begins to rise in one of the side tubs and falls in the other.
-
The phenomenon can be explained as follows :
We have already seen that the colloidal particles and dispersion medium carry charges which are equal but opposite in nature. Under the influence of an electric field, both have a tendency to move towards the oppositely charged electrodes. Semi permeable membranes do not allow the passage of colloidal particles. However, dispersion medium can pass through them. Therefore during electro-osmosis, colloidal particles are checked and it is the dispersion medium that moves towards the oppositely charged electrode.
Coagulation or Flocculation
-
The stability of a sol is due to the charge present on the colloidal particles. Due to similar charges, colloidal particles repel one another and are unable to combine together to form larger particles. However, if the charge on colloidal particles is destroyed, they are free to come nearer and grow in size.
-
When the particles become sufficiently large, they get precipitated. This phenomenon is termed as coagulation or flocculation.
-
The coagulation of colloidal solution can be achieved by the addition of an electrolyte.
-
It is to be noted that a small amount of electrolyte is necessary for the stability of a sol because the ions of the electrolyte get adsorbed on colloidal particles and impart them some charge. However, when an electrolyte is added in substantial amount the positively charged ions of the electrolyte neutralize the charge on colloidal particles and compel the sol to get coagulated.
-
Coagulation may be defined as the phenomenon involving the precipitation of a colloidal solution on addition of an electrolyte.
-
Hardy-Schulze rule: The coagulation capacity of an electrolyte depends upon the valence of ion responsible for causing coagulation. As we have seen above, the ion responsible for causing coagulation is the one which carries charge opposite to that present on colloidal particles. For example, a positively charged sol gets coagulated by the negatively charged ions of the added electrolyte. From a study of the coagulation behavior of various electrolytes towards a particular sol, Hardy and Schulze suggested a general rule known as Hardy-Schulze rule.
The rule can be stated as the greater is the valence of the oppositely charged ion of the electrolyte added to a colloidal solution, the faster is the coagulation of the colloidal solution. -
Thus, higher the charge on oppositely charged ion greater is its coagulating power. For example, the coagulation power of different cations for coagulating a negatively charged sol of As2S3 follows the order.
Al3+ > Ba2+ > Na+ -
Similarly, for the coagulation of a positively charged sol such as Fe(OH)3, the coagulating power of different anions follows the order.
[Fe(CN)6]4- > PO43- > SO42- > Cl- -
Flocculation value: The coagulating power of an electrolyte is usually expressed in terms of its flocculation value which may be defined as the minimum concentration (in millimoles per litre) of an electrolyte required to cause the coagulation of a sol.
-
The flocculation values (in millimoles per litre) for the coagulation of negatively charged As2S3 sol and positively charged Fe(OH)3 sol are given in the Table below.
|
For Negatively charged As2S3 Sol |
For Positively charged Fe(OH)3 Sol |
||||
|
Electrolyte |
Flocculating ion |
Flocculation value (millimoles/litre) |
Electrolyte |
Flocculating ion |
Flocculation value (millimoles/litre) |
|
NaCl |
Na+ |
52 |
KBr |
Br- |
138 |
|
KCl |
K+ |
50 |
HCl |
Cl- |
132 |
|
HCl |
H+ |
30 |
KNO3 |
NO-3 |
132 |
|
MgCl2 |
Mg2+ |
0.72 |
K2CrO4 |
CrO42- |
0.315 |
|
BaCl2 |
Ba2+ |
0.69 |
K2SO4 |
SO42- |
0.210 |
|
ZnCl2 |
Zn2+ |
0.68 |
K2C2O4 |
C2O42- |
0.238 |
|
AlCl3 |
Al3+ |
0.093 |
K3[Fe(CN)6] |
Fe(CN)6]3- |
0.096 |
It is to be noted that a smaller flocculation value indicates the greater coagulating power of the electrolyte. Thus.

Some other methods for causing coagulation: The most commonly used method for causing coagulation in a colloidal solution is the addition of an electrolyte as described above. However, the coagulation of colloidal solution can also be achieved by any of the following methods.
-
By electrophoresis: In electrophoresis, the charged colloidal particles migrate to the oppositely charged electrode and get discharged. This results in the coagulation of the colloidal solution.
-
By mixing two oppositely sols: When two sols carrying opposite charges are mixed together in suitable proportions, the colloidal particles of one sol neutralize the charge present on the particles of the other sol and both get coagulated.
-
By persistent dialysis: We have already seen that a small amount of electrolyte is essential to make a sol stable. When a sol is subjected to persistent dialysis, the traces of electrolyte also pass out through the membrane. In the absence of electrolyte, sol becomes unstable and gets coagulated.??
Protective Colloids and Gold Number
-
Lyophobic sols such as those of metals (e.g. Au, Ag, Pt etc.) are not very stable in the sense that they get easily coagulated (precipitated) in the presence of an electrolyte. This poses a big problem in their storage and usage. Contrary to this, lyophilic sols are much more stable and do not get coagulated easily under similar conditions.
-
It has been observed that in the presence of certain lyophilic colloids such as gum Arabic, gelatin, starch etc. the hydrophobic sols acquire greater stability towards coagulation, i.e. they get protected and do not get coagulated easily when an electrolyte is added.
-
The process of protecting a lyophobic sol from being coagulated (precipitated) on addition of an electrolyte by the use of a lyophilic colloids is called protection and the lyophilic colloid used for purpose is called a protective colloid.
For example, the addition of gelatin (a lyophilic colloid) to a gold sol (lyophobic sol) protects the latter from being coagulated on addition of sodium chloride solution. -
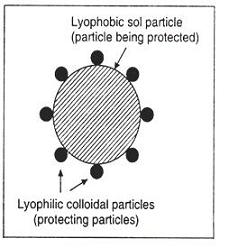 The exact mechanism of protection is not very clearly understood. However, it is believed that the lyophilic colloid particles get adsorbed on the surface of the colloid particles present in the lyophobic sol. The adsorbed lyophilic particles thus form an envelope around the lyophobic sol particles and protect them from the action of electrolytes.
The exact mechanism of protection is not very clearly understood. However, it is believed that the lyophilic colloid particles get adsorbed on the surface of the colloid particles present in the lyophobic sol. The adsorbed lyophilic particles thus form an envelope around the lyophobic sol particles and protect them from the action of electrolytes. -
Gold Number: The protective power a lyophilic colloid is usually expressed in terms of a number called gold number introduced by Zsigmondy (1901).. The gold number of a protective colloid is its minimum amount in milligrams which is just sufficient to prevent the coagulation of 10 ml of a gold sol on the addition of 1 mL of 10% sodium chloride solution.
-
It is to be noted that the smaller the value of gold number, the greater is the protective power of the protective colloid. The gold numbers of a few protective colloids are given in the Table below.
|
Protective (Lyophilic) Colloid |
Gold Number |
|
Gelatin |
0.005-0.01 |
|
Casein |
0.01-0.02 |
|
Hemoglobin |
0.03-0.07 |
|
Egg albumin |
0.08-0.10 |
|
Gum Arabic |
0.10-0.15 |
|
Dextrin |
6-20 |
|
Starch |
20.25 |

Question 1: Which of the following alternatives represents the correct order of coagulation power of ions?
a. Na+>Al3+ > Ba2+
b. Ba2+ > Al3+ > Na+
c. Ba2+ > Na+ >Al3+
d. Al3+ > Ba2+ > Na+
Question 2: When an intense converging beam of light is passed through a colloidal solution kept in dark, the path of the beam gets illuminated with a bluish light. This phenomenon is called
a. Tyndrall effect
b. Coagulation of colloids
c. Peptization
d. Dispersion of light
Question 3: Which of the following alternatives does not form a positively charged sol ?
a. Fe(OH)3
b. Al(OH)3
c. TiO2
d. Cd
Question 4: The minimum amount of any colloid in milligrams which is just sufficient to prevent the coagulation of 10 ml of a gold sol on the addition of 1 mL of 10% sodium chloride solution is called its
a. gold number
b. coagulation value
c. flocculation value

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
d |
a |
d |
a |
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Click here access the syllabus of chemistry for IIT JEE
-
Click here for colloidal state
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More