Solved Examples on Surface Chemistry
Question 1:
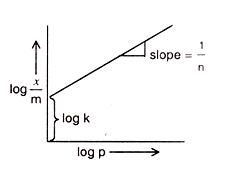
For Adsorption of a gas on a solid, the plot of log x/m vs
lop p is linear with slope equal to (n being whole number)
a. K
b. log K
c. n
d. 1/n
Answer : 1/n
Solution:
At low pressures : x/m varies linearly with p

or 
At high pressures : x/m is independent of p

At intermediate pressures: The variation of x/m vs p can be expressed as

where
n > 1.
or

or

Comparing the above given equation with equation of straight line
y = mx + c
we know that, if we plot log p vs log x/m, we would get a straight line with slope equal to 1/n and intercept log k.
________________________________________________________________
Question 2:
Which of the following statements regarding the physical adsorption of a gas on surface of a solid is not correct?
a. On increasing temperature, adsorption increases continuosly.
b. Enthalpy changes are negative.
c. Adsorption is more for some specific substances.
d. It is reversible in nature.
Answer : a
Solution: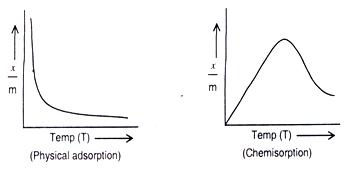
As adsorption is accompanied by release of heat energy, so in accordance with Le-Chatelier’s principle, the increase of temperature should decrease the extent of adsorption. This has indeed been found to be so. A plot of x/m vs. temperature at constant pressure is called adsorption isobar. In the case of physical adsorption x/m decreases with increase of temperature. However, in the case of chemisorption x/m initially increases with temperature and then decreases as shown below. The initial increase is due to the fact that chemisorptions require activation energy.
_________________________________________________________
Question 3:
Which of the following is not characteristic of chemisorption?
a. It is irreversible.
b. It is specific
c. It is multilayer phenomenon
d. Heat of adsorption is about 400 kj
Answer: c.
Solution:
Chemisorption involves formation of chemical bonds between adsorbate and adsorbent molecules. Once the valency is satisfied, the adsorbent molecules can’t form bond with more adsorbate molecules. Thus only one layer is formed.
? __________________________________________________________________
Question: 4
Rate of physisorption increases with
a. decrease in temperature
b. increase in temperature
c. decrease in pressure
d. decrease in surface area
Answer: a
Solution: In physisorption, adsorbate is held on the surface of adsorbent by van der Waals forces only. The attraction between surface of adsorbent and the absorbate particles decreases with increase in temperature and vice versa.
Hence, the correct option is A.
? _______________________________________________________________________________
Question 5:
Elaborate the process of dialysis and electrodialysis of colloids.
Solution:
Dialysis and electrodialysis are the methods for purification of colloids.
-
Dialysis is the process of separating the impurity particles of true solution dimensions (crystalloids) from an impure sol by means of diffusion through a suitable membrane such as parchment paper or cellophane membrane is called dialysis.

The apparatus used in this method is called dialyser. It consists of a bag made of parchment or cellophane. The bag is filled with the impure sol to be purified and is suspended in a tank through which pure water is circulated. The impurities of electrolytes present in the sol diffuse out of the bag leaving behind pure sol in the bag. -
Electrodialysis: Dialysis is a slow process. However, it can be expedited by applying an electric field. Under the influence of electric field, the impurity ions move faster to the oppositely charged electrodes and the process gets quickened. This process is referred to as electrodialysis.

______________________________________________________________________
Question 6:
For the coagulation of 100 mL of arsenious sulphide sol, 5 ml of 1 M NaCl is required. What is the flocculation value of NaCl?
Solution:
5 mL of 1 M NaCl contains NaCl = (1/1000) x 5 moles = 5 millimoles
Thus, 100 mL of As2S3 sol require NaCl for complete coagulation = 5 millimoles
so, 1 L, i.e., 1000 mL of the sol require NaCl for complete coagulation = 50 millimoles
so, by definition, flocculation value of NaCl = 50.
____________________________________________________________________________
Question 7:
Explain the following
a. Tyndral Effect
b. Brownian Movement
Solution:
a. Tyndall Effect:
When an intense converging beam of light is passed through a colloidal solution kept in dark, the path of the beam gets illuminated with a bluish light. This phenomenon is called Tyndall effect and the illuminated path is known as Tyndall cone. The phenomenon was first observed by Tyndall in 1869.

The Tyndall effect is due to the scattering of light by colloidal particles. Since the dimensions of colloidal particles are comparable to the wavelength of ultraviolet and visible radiations, they scatter these radiations and get illuminated.
Tyndall observed that the zone of scattered light is much larger than the particle itself. This is why colloidal particles look like bright spots when viewed with a microscope at right angles to the beam of light as shown in figure. Thus, Tyndall effect may be defined as the scattering of light by colloidal particles present in a colloidal solution.
Tyndall effect is not exhibited by true solutions. This is because the particles (ions or molecules) present in a true solution are too small to scatter light. Thus,
Tyndall effect can be used to distinguish a colloidal solution from a true solution. The phenomenon has also been used to devise an instrument known as ultra microscope. The instrument is used for the detection of the particles of colloidal dimensions. Tyndall effect also establishes the fact that colloidal systems are heterogeneous in nature.
b. Brownian Movement

Colloidal particles present in a colloidal solution exhibit a very important property called Brownian movement. When a colloidal solution is viewed under an ultra microscope, the colloidal particles are seen continuously moving in a zigzag path. The property was discovered by a botanist Robert Brown in 1827, when he observed that pollen grains suspended in water exhibit random zigzag motion. After the name of the discoverer, the property was named as Brownian movement. It may be defined as follows.
The continuous zigzag movement of the colloidal particles in the dispersion medium in a colloidal solution is called Brownian movement.
_____________________________________________________________________________
Question 8:
Which of the following electrolytes is most effective coagulating agent for Sb2S3?
a. Na2SO4
b. CaCl2
c. Al2(SO4)3
d. NH4Cl
Answer: c
Solution: As Sb2S3 is a negative sol, so will be the most effective coagulating agent due to higher positive charge at Al (Al3+).
Hence, the correct option is C.
_______________________________________________________________________
Question 9:
Exaplain the ?mechanism of enzyme catalysis.
Solution:
Two models of enzyme action have been proposed.
-
Lock and key hypothesis
-
Induced fit hypothesis
According to the lock-and-key model, when the ‘key (substrate) fits the ‘lock’ (active site), the chemical change begins. Lock and key model for enzymatic catalysis was first postulated in 1894 by Emil Fischer.
The lock and key theory can be explained easily by the fact that a particular lock can be opened by a particular key specially designed to open it. Similarly enzymes have specific sites where a particular substrate can only be attached. The lock and key model accounts for enzyme specificity

However, modern X-ray crystallographic and spectroscopic methods show that in many cases, the enzyme changes shape when the substrate lands at the active site. This induced-fit model of enzyme action pictures the substrate inducing the active site to adopt a perfect fit, rather than a rigidly shaped lock and key. Therefore, we might picture a hand in a glove, in which the ‘glove’ (active site) does not attain its functional shape until the ‘hand’ (substrate) moves into place.
The kinetic of enzyme catalysis has many features in common with ordinary catalysis. In the enzyme catalyzed reaction, substrate (S) and enzyme (E) form an intermediate enzyme-substrate complex (ES) whose concentration determines the rate of product (P) formation. The steps common to virtually all enzyme catalyzed reactions are:


The rate of enzyme catalyzed reaction changes from first-order to zero-order as the concentration of substrate is increased.
The induced fit model assumes that the substrate plays a role in determining the final shape of the enzyme and that the enzymes are partially flexible. This explains why certain compounds can bind to the enzyme but do not react because the enzyme has been distorted too much. Other molecules may be too small to induce the proper alignment and therefore cannot react. Only the proper substrate is capable of inducing the proper alignment of the active site.
_____________________________________________________________________________________
Question 10:
Among the following, the surfactant that will form micelles in aqueous solution at lowest molar concentration at ambient condition is-
a. CH3(CH2)15N(CH3) 3Br
b. CH3(CH2)11OSO3Na
c. CH3(CH2)6COONa
d. CH3(CH2)11N(CH3) 3Br
Answer: a
Solution: Critical concentration for micelle formation decreases as the molecular weight of hydrocarbon chain of surfactant grows because in this case true solubility diminishes and the tendency of surfactant molecules to associate increases.
Hence, the correct option is A.
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Click here access the syllabus of chemistry for IIT JEE
-
Click here for colloidal state
To read more, Buy study materials of Surface Chemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More
