Theory of Indicators
Table of Content |

 An indicator is a substance which show characteristic change in its colour when comes in contact with acid or base and thus it is used to determine dicators the degree of acidity or basicity of any solution. For example litmus solution or litmus paper.
An indicator is a substance which show characteristic change in its colour when comes in contact with acid or base and thus it is used to determine dicators the degree of acidity or basicity of any solution. For example litmus solution or litmus paper.
Role of indicators in chemistry is very important. They are used are also used to find out the end point in a titration.
In acid-base titrations, organic substances (weak acids or weak bases) are generally used as indicators.
Indicators change their colour within a certain pH range.The colour change and the pH range of some common indicators used are tabulated below:
There are two theories which explain the change of colour indicators with change in pH.
Ostwald's Theory
According to Ostwald’s theory
-
The colour change of any indicator is due to its ionisation. The unionised form of indicator has different colour than its ionised form.
-
An inidicator is either a weak acid or base, so its ionisation is highly affected in acids and bases. If an indicator is a weak acid, its ionisation would be very much low in acids due to common H+ ions while it is fairly ionised in alkalies. In the same way, if the indicator is a weak base, its ionisation is large in acids and low in alkalies due to common OH- ions.
Let’s take examples of two important indicators phenolphthalein which is a weak acid and methyl orange which is a weak base.
1. Phenolphthalein
It is represented as HPh. This indicator being a weak acid ionises in solution to a small extent as follows:
HPh  H+ + Ph-
H+ + Ph-
Colourless Pink
Applying law of mass action, we get
K = [H+][Ph-]/[HPh]
The undissociated molecules of phenolphthalein are colourless while the Ph- ions are pink in colour. In presence of an acid , ionisation of HPh is practically negligible as the equilibrium shifts to left hand side due to high concentration of H+ ions. Thus, the solution would remain colourless. On addition of alkali, hydrogen ions are removed by OH- ions in the form of water molecules and the equilibrium shifts to right hand side. Thus, the concentration of Ph- ions increases in solution and they impart pink colour to the solution.
2. Methyl Orange
It is a very weak base and can be represented as MeOH. It is ionized in solution to give Me+ and OH- ions.
MeOH  Me+ + OH-
Me+ + OH-
Yellow Red
Applying law of mass action,
K = [Me+ ][OH- ]/[MeOH]
In presence of an acid, OH- ions are removed in the form of water molecules and the above equilibrium shifts to right hand side. Thus, sufficient Me+ ions are produced which impart red colour to the solution. On addition of alkali, the concentration of OH- ions increases in the solution and the equilibrium shifts to left hand side, i.e., the ionisation of MeOH is practically negligible. Thus, the solution acquires the colour of unionised methyl orange molecules, i.e., yellow.
This theory also explains the reason why phenolphthalein is not a suitable indicator for titrating a weak base against strong acid. The OH- ions furnished by a weak base are not sufficient to shift the equilibrium towards right hand side considerably, i.e., pH is not reached to 8.3. Thus, the solution does not attain pink colour. Similarly, it can be explained why methyl orange is not a suitable indicator for the titration of weak acid with strong base.
|
There are substances present in our kitchen which can be used as acid-base indicators..
|
-
Quinonoid theory
According to quinonoid theory, an acid-base indicators exist in two tautomeric forms having different structures which are in equilibrium. One form is termed benzenoid form and the other quinonoid form.
The two forms have different colors. The color change is due to the interconversation of one tautomeric form into other. One form mainly exists in acidic medium and the other in alkaline medium.
Thus, during titration the medium changes from acidic to alkaline or vice-versa. The change in pH converts one tautomeric form into other and thus, the colour change occurs.
Phenolphthalein has benziod form in acidic medium and thus, it is colourless while it has quinonoid form in alkaline medium which has pink colour.
Methyl orange has quinonoid form in acidic solution and benzenoid form in alkaline solution. The color of benzenoid form is yellow while that of quinoniod form is red.
Refer to the following video for acid-base indicators
-
Selection of Suitable Indicator or Choice of Indicator
Incorrect detection of end point will affect the titration calculation and thus it becomes very important to select the correct indicator used in titration. The neutralisation reactions are of the following four types:
-
A strong acid versus a strong base.
-
A weak acid versus a strong base.
-
A strong acid versus a weak base.
-
A weak acid versus a weak base.
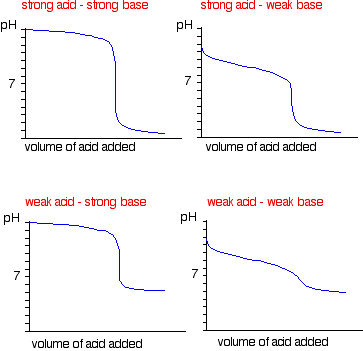
In order to choose a suitable indicator, it is necessary to understand the pH changes in the above four types of titrations. The change in pH in the vicinity of the equivalence point is most important for this purpose. The curve obtained by plotting pH as ordinate against the volume of alkali added as abscissa is known as neutralisation or titration curve. The titration curves of the above four types of neutralisation reactions are shown in Fig. 10.1, 10.2, 10.3 and 10.4.
In each case 25 mL of the acid (N/10) has been titrated against a standard solution of a base (N/10). Each titration curve becomes almost vertical for some distance (except curve 10.4) and then bends away again. This region of abrupt change in pH indicates the equivalence point. For a particular titration, the indicator should be so selected that it changes its colour within vertical distance of the curve.
(i) Strong acid vs. strong base
pH curve of strong acid (say HCI) and strong base (say NaOH) is vertical over almost the pH range 4-10. So the indicators phenolphthalein (pH range 8.3 to 10.5), methyl red (pH range 4.4-6.5) and methyl orange (pH range 3.2-4.5) are suitable for such a titration.
(ii) Weak acid vs. weak base:
pH curve of weak acid (say CH3COOH of oxalic acid) and strong base (say NaOH) is vertical over the approximate pH range 7 to 11. So phenolphthalein is the suitable indicator for such a titration.
(iii) Strong acid vs. weak base:
pH curve of strong acid (say HCl or H2SO4 or HNO3) with a weak base (say NH4OH) is vertical over the pH range of 4 to 7. So the indicators methyl red and methyl orange are suitable for such a titration.
(iii) Weak acid vs. weak base:
pH curve of weak acid and weak base indicates that there is no vertical part and hence, no suitable indicator can be used for such a titration.
(iii) Titration of soluble carbonate with strong acid.
pH curve of sodium carbonate with HCI shows two inflection points (Fig. 10.5). First inflection point (pH 8.5) indicates conversion of carbonate into bicarbonate.
Na2CO3 + HCI → NaHCO3 + NaCl
As the inflection point lies in the pH range 8 to 10, phenolphthalein can be used to indicate the above conversion. The second inflection point (pH 4.3) indicates the following reaction:
NaHCO3 + HCI → NaCl + CO2 + H2O
As the point lies between 3 to 5, methyl orange can be used.

Question 1: Which of the following can not be used as an acid-base indicator?
a. Methyle orange
b. Curcumin
c. Indego
d. Beet
Question 2: Which of the following acid-base indicators give red colour in basic medium?
a. Methyl Red
b. Alizarin Yellow
c. Phenolphthalein
d. Bromothymol blue
Question 3: ___ is colourless in acidic solutions.
a. Methyl Red
b. Alizarin Yellow
c. Phenolphthalein
d. Bromothymol Blue
Question 4: pH range for litmus is ..
a. 5.5-7.5
b. 4.9-9.5
c. 7.1-8.2
d. 1.2-14.3
Question 5: Which of the following acid-base indicators will not change the colour of dilute HCl solution?
a. Methyl Yellow
b. Alizarin Yellow
c. Phenolphthalein

| Q.1 | Q.2 | Q.3 | Q.4 | Q.5 |
| c | d | c | a | c |
Related Resources
To read more, Buy study materials of Chemical Equilibrium comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.