Isomerism in Coordination Compounds
Compounds that have the same chemical formula but different structural arrangements are called isomers. Due to their complicated formulae of many coordination compounds, the variety of bond types and the number of shapes possible, many different types of isomerism occur.

Polymerization Isomerism:
This is not true isomerism because it occurs between compounds having the same empirical formula, but different molecular weights.
-
[Pt(NH3)2Cl2],
-
[Pt(NH3)4][PtCl4]
-
[Pt(NH3)4] [Pt(NH3)Cl3]2.
Ionization Isomerism:
This type of isomerism is due to the exchange of groups between the complex ion and the ions outside it. [Co(NH3)5Br]SO4 is red – violet. An aqueous solution gives a white precipitate of BaSO4 with BaCl2 solution, thus confirming the presence of free SO42- ions. In contrast [Co(NH3)5SO4]Br is red. A solution of this complex does not give a positive sulphate test with BaCl2. It does give a cream – coloured precipitate of AgBr with AgNO3, the confirming the presence of free Br– ions.
Hydrate Isomerism:
Three isomers of CrCl3.6H2O are known. From conductivity measurements and quantitative precipitation of the ionized chlorine, they have been given the following formuale:
-
[Cr(H2O)6]Cl3 : violet (three ionic chlorines)
-
[Cr(H2O)5Cl]Cl2.H2O : green (two ionic chlorines)
-
[Cr(H2O)4Cl2].Cl.2H2O : dark green (one ionic chlorine)
Linkage Isomerism:
Certain ligands contain more than one atom which could donate an electron pair. In the NO2- ion, either N or O atoms could act as the electron pair donor. Thus there is the possibility of isomerism.
Two different complexes [Co(NH3)5NO2]Cl2 have been prepared, each containing the NO2- group in the complex ion.
Geometric isomerism:
In disubstituted complexes, the substituted groups may be adjacent or opposite to each other. This gives rise to geometric isomerism. Thus square planar complexes such as [Pt(NH3)4Cl2] can be prepared in two forms, cis and trans. When the chlorine atoms are adjacent to each other it is called cis form. While when two chlorine atoms are opposite it is called transform.
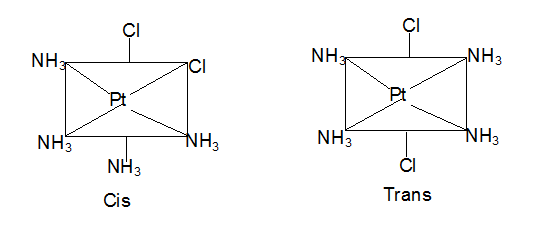
-
This type of isomerism takes place mainly in heteroleptic complexes because of the different possible geometric arrangements of ligands around the central metal atom.
-
This type of isomerism is mainly found in coordination compounds with coordination numbers 4 and 6.
-
In a square planar complex (i.e. coordination compounds with coordination number 4 which have [MX2L2] type formula (X and L are unidentate ligands), the two ligands X may be present adjacent to each other in a cis isomer, or opposite to each other to form a trans isomer.
![In a square planar complex (i.e. coordination compounds with coordination number 4 which have [MX2L2] type formula (X and L are unidentate ligands), the two ligands X may be present adjacent to each other in a cis isomer, or opposite to each other to form a trans isomer](https://files.askiitians.com/cdn1/images/2015514-171655888-5946-untitled.png)
-
Square planar complex with MABXL type formula (where A, B, X, L are unidentate ligands) show three isomers-two cis and one trans.
-
Sis trans isomerism is not possible for a tetrahedral geometry.
-
But octahedral complexes do show cis trans isomerism. In complexes with formula [MX2L4] type, two ligands X may be oriented cis or trans to each other.
-
This type of isomerism is also observed when bidentate ligands L–L [e.g.,NH2 CH2 CH2 NH2 (en)] are present in complexes with [MX2(L–L)2] type formula
-
There is another type of geometrical isomerism which occurs in octahedral coordination entities with [Ma3b3] type formula. Examples is [Co(NH3)3(NO2)3].
-
If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer.
-
When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer
-
Facial Isomers: A set of three ligands (similar) arranged on an octahedron in all cis – fashion.
-
Meridional Isomers: A set of three similar ligands arranged on an octahedron with one pair trans

Refer to the following video for isomerism in coordination compounds
Optical Isomerism
-
¨The complexes which are non-superimposable on their mirror images are optically active.
-
Optically active complexes are asymmetric in nature i.e. not divisible into two identical halves.
-
Levorotatory (l) – the compound which rotates plane polarised light to left hand side.
-
Dextrorotatory (d) – the compound which rotates plane polarised light to right hand side
-
d and l isomers of a compound are called enantiomers
-
-
Octahedral complexes with coordination number 6 involving 2 or 3 bidentate ligands show optical isomerism.
|
Solved Problem |
|
Question1: How will you distinguish between the following isomer pairs a) [CoBr(NH3)5]SO4 and [Co(SO4) (NH3)5]Br b) [Cr(H2O)6]Cl3 and [CrCl(H2O)5]Cl2H2O c) Cis [PtCl2(NH3)2] and trans[PtCl2(NH3)2] d) The two entiomers of [CoCl2(en)2]+ Solution: a) Isomer (i) gives white ppt of BaSO4 with BaCl2 whereas isomer (ii) does not form a ppt. b) The water molecule in isomer (ii) is lost easily on heating whereas the water molecule in isomer (i) are not lost easily, being coordinated to the central atom. c) Cis isomer (i) has dipole moment, the trans isomer (ii) does not d) One isomer is dextrorotory whereas the other is laevorotatory. _____________________________________________________________ Question 2: The geometry of Ni(CO)4 and Ni(PPH3)2Cl2 are (a) both square planar (b) tetrahedral and square planar, respectively (c) both tetrahedral (d) square planar and tetrahedral, respectively Solution : c
________________________________________________________________________ Question 3: The ionization isomer of [Cr(H2O)4Cl(NO2)]Cl is (a) [Cr(H2O)4(O2N)]Cl2 (b) [Cr(H2O)4Cl2](NO2) (c) [Cr(H2O)4Cl(ONO)]Cl (d) [Cr(H2O)4Cl2(NO2)]∙H2O Solution: b Ionisation isomers are the complexes that produces different ions in solution, ie, they have ions interchanged inside and outside the coordination sphere. [Cr(H2O)4 Cl(NO2)]Cl and [Cr(H2O)4 Cl2] (NO2) have different ions inside and outside the coordinate sphere and they are isomers. Therefore, they are ionization isomers. ______________________________________________________________________ Question 4: The compound(s) that exhibit(s) geometrical isomerism is(are) (a) [Pt(en)Cl2] (b) [Pt(en)2]Cl2 (c) [Pt(en)2Cl2]Cl2 (d) [Pt(NH3)2]Cl2 Solution: c and d Both [Pt(en)2 Cl2]Cl2 and [Pt(NH3)2 Cl2] are capable of showing geometrical isomerism.
|
Related Resources
-
You can also refer to our exam section to download the past year papers of IIT JEE and other exams
-
Click here to download the NCERT Solutions
-
Refer to the nomenclature of coordination compounds to know how to name them.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

![[Pt(en)2 Cl2]Cl2 and [Pt(NH3)2 Cl2] are capable of showing geometrical isomerism.](https://files.askiitians.com/cdn1/images/2015514-174327545-6375-capture.png)