d and f-Block Elements
What are transition elements?
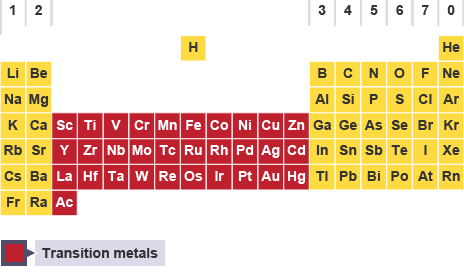
The elements which are present between s and p-block elements in the modern periodic table are called transition elements.Transition elements have partly filled (n-1) d-orbitals.
In transition elements the last electron enters penultimate d orbitals i.e. (n-1)d orbitals and that is why they are called d-block elements.
The general valence shell configurations of transition elements is (n-1)d1–10.ns0, 1, 2.
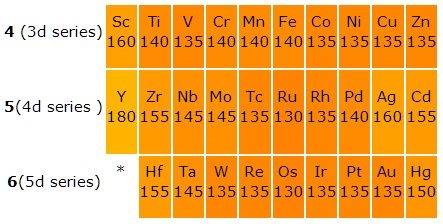
All the d-block elements are classified into four series viz 3d, 4d, 5d and 6d series corresponding to the filling of 3d, 4d, 5d and 6d orbitals.
We will gain in depth knowledge of thransition metals under following subtopics:
What are f block elements?
f –block elements are also called inner transition elements. In these the last electron enters penultimate i.e. (n – 2)f f orbital. The differentiating electron in transition elements may enter either 4f or 5f orbitals based upon which they are differentiated into lanthanides and actinides.
Lanthanides: In lanthanides the differentiating electron enters 4f orbital. These are cerium to lutetium. The name lanthanides is because they come immediately after lanthanum.
Actinides: In actinides the differentiating electron enters 5f orbitals. These are thorium to lawrencium. These elements come immediately after actinium.
Electronic configuration: General electronic configuration of f – block elements is (n–2)f1–14(n–1)d0–1ns2
-
Lanthanides: [Xe]4f1–145d0–16s2
-
Actinides: [Rn]5f1–146d0–17s2
Refer to the following video for electronic configuration of transition elements
Related Resources
-
Click here for past year papers of IIT JEE
-
You can also refer to the syllabus of chemistry for IIT JEE
-
Knowing the about the important books for preparing IIT JEE would help you a lot.