Lanthanides and Actinides
Table of Content |
Lanthanides
Lanthanides consist of elements that follow lanthanum and involve the filling of 4f subshell
Electronic Configuration
[Xe] 4fn+1 5d° 6s2 or [Xe] 4fn 5d1 6s2
The general valence shell electronic configuration of lanthanides is 4f1−146s2.
Electronic configurations of lanthanum and lanthanides are listed in the table
|
Atomic Number |
Name |
Symbol |
Electronic configurations |
Radii/pm |
||||
|
Ln |
Ln2+ |
Ln3+ |
Ln4+ |
Ln |
Ln3+ |
|||
|
57 |
Lanthanum |
La |
5d16s2 |
5d1 |
4f0 |
- |
187 |
106 |
|
58 |
Cerium |
Ce |
4f15d16s2 |
4f2 |
4f1 |
4f0 |
183 |
103 |
|
59 |
Praseodymium |
Pr |
4f36s2 |
4f3 |
4f2 |
4f1 |
182 |
101 |
|
60 |
Neodymium |
Nd |
4f46s2 |
4f4 |
4f3 |
4f2 |
181 |
99 |
|
61 |
Promethium |
Pm |
4f56s2 |
4f5 |
4f4 |
- |
181 |
98 |
|
62 |
Samarium |
Sm |
4f66s2 |
4f6 |
4f5 |
- |
180 |
96 |
|
63 |
Europium |
Eu |
4f76s2 |
4f7 |
4f6 |
- |
199 |
95 |
|
64 |
Gadolinium |
Gd |
4f75d16s2 |
4f35d1 |
4f7 |
- |
180 |
94 |
|
65 |
Terbium |
Tb |
4f96s2 |
4f9 |
4f8 |
4f7 |
178 |
92 |
|
66 |
Dysprosium |
Dy |
4f106s2 |
4f10 |
4f9 |
4f8 |
177 |
91 |
|
67 |
Holmium |
Ho |
4f116s2 |
4f11 |
4f10 |
- |
176 |
89 |
|
68 |
Er |
4f126s2 |
4f12 |
4f11 |
- |
175 |
88 |
|
|
69 |
Thulium |
Tm |
4f136s2 |
4f13 |
4f12 |
- |
174 |
87 |
|
70 |
Ytterbium |
Yb |
4f146s2 |
4f14 |
4f13 |
- |
173 |
86 |
|
71 |
Lutetium |
Lu |
4f145d16s2 |
4f145d1 |
4f14 |
- |
- |
- |
Atomic and Ionic Sizes of Lanthanides
-
Atomic and ionic radii of lanthanides decrease with an increase in atomic number. This gradual decrease is known as lanthanides contraction.
-
Because of the lanthanides contraction, the radii of the elements of the 3rd transition series are very similar to those of the corresponding elements of the 2nd transition series elements.
Oxidation States of Lanthanides
Lanthanides exhibit the oxidation state of +3. Some of them also exhibit the oxidation state of +2 and +4.
-
a noble gas configuration e.g. Ce4+ (f0)
-
a half filled f shell e.g. Eu2+ (f7)
-
a completely filled f shell e.g. YB22+ (f14)
Lanthanide contraction
It is observed that in lanthanide series, there is a progressive decrease in the atomic and ionic radii with increasing atomic number . This regular decrease with increase in atomic number is called lanthanide contraction. This is due to the weak shielding of f orbitals. These f orbitals are unable to counter balance the effect of increasing nuclear charge because of which the size keeps on decreasing with increase in atomic umber.
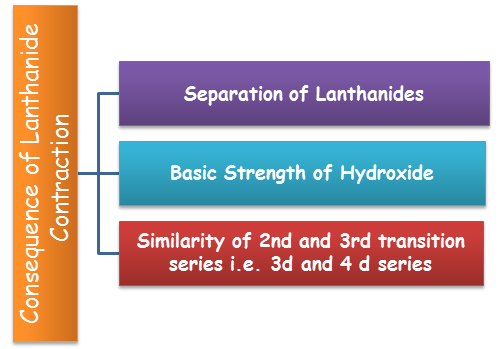
Causes of Lanthanide Contraction: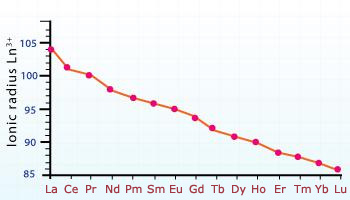
As we move along the period from left to right in lanthanide series, the atomic number increases i.e. number of protons keeps on increasing.For every proton added in the nucleus the extra electron goes to the same 4f orbital.
The 4f orbital shows poor shielding effect because of which there is a gradual increase in the effective nuclear charge experienced by the outer electrons. Thus, the attraction of the nucleus for the electrons in the outermost shell increases in atomic number.
Consequence of Lanthanide Contraction
-
Separation of Lanthanides: Without lanthanide contraction all the lanthanides would have same size because of which if would have been very difficult to separate them but due to lanthanide contraction their properties slightly vary. The variation in the properties is utilized for separating them.
-
Basic Strength of Hydroxide: Because of the lanthanide contraction, size of M3+ ions decreases and there is increase in covalent character in M–OH and hence basic character decreases.
-
Similarity of 2nd and 3rd transition series i.e. 3d and 4 d series: The atomic sizes of second row transition elements and third row transition elements are almost similar. This is also an effect of lanthanide contraction. As we move down the from form 4d to 5d series, the size must increase but it remains almost same due to the fact that the 4f electrons present in the 5d elements show poor shielding effect.
Refer to the following video for f block elements
Complex formation
-
The lanthanides do not show much tendency to form complexes due to low charge density because of their size. However, the tendency to form complex and their stability increases with increasing atomic number.
Chemical Behaviour
The first few members of the series are quite reactive like calcium. However with increasing atomic number, their behaviour becomes similar to that of aluminum.
-
Lanthanides combine with hydrogen on gentle heating. When they are heated with carbon result in formation of carbides. On burning in the presence of halogens, lanthanides form halides.
-
Lanthanides react with dilute acids to liberate hydrogen gas.
-
Lanthanides form oxides and hydroxides of the type N2O3 and M(OH)3 which are basic alkaline earth metal oxides and hydroxides.
Uses of Lanthanides
-
Lanthanide are used in the production of alloy steels for plates and pipes.
-
Mixed oxides of lanthanides are used as catalysts in petroleum cracking industries.
-
Some lanthanum oxides are used as phosphors in television screens and other fluorescing surfaces.
Actinides
Actinides consist of elements that follow actinium and involve the filling of 5f subshell .
Electronic Configuration
[Rn] 5f0-146d0-2 7s2
Oxidation States
The dominant oxidation state of these elements is +3 (similar to lanthanides). Besides +3 state, they also exhibit +4 oxidation state. Some actinides show still higher oxidation states. The maximum oxidation state first increases upto the middle of the series and then decreases i.e. it increases from +4 for Th to +5, +6 and +7 for Pa, V and Np but decreases in the succeeding elements.
Melting and boiling point
They have high melting and boiling points like lanthanides but don’t show any regular trend with increasing atomic number.
Density:
All actinides except thorium and amercium have high density.
Ionization enthalpies:
The actinides have lower ionization enthalpies as comapre to lanthanides because 5f is more effectively shielded from nuclear charge than 4f.
Magnetic behavior:
All actinides are paramagnetic in nature. The paramagnetic nature which depends on the presence of unpaired electrons.
Radioactivity:
All the actinides are radioactive in nature. Radioactivity increases with increase in atomic number.
Chemical Behaviour
The ability of actinides to exist in different oxidation states has made their chemistry more complex. Moreover, most of these elements are radioactive and the study of their chemistry in the laboratory is difficult.
-
They react with boiling water to give a mixture of oxide and hydride.
-
The combine with most of the non – metals at moderate temperature.
-
All these metals are attacked by HCl but the effect of HNO3 is very small due to the formation of a protective oxide layer on their surface.
Related Resources
-
Download the past year papers of IIT JEE
-
Have a look at the syllabus of chemistry for IIT JEE
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free