Dielectrics and Polarisation
Table of Content |
Introduction to Dielectrics and Polarisation
This topic deals with the content on Dielectric and Polarisation. What are dielectrics and their types that is, polar and non-polar molecules, what is Polarisation, polarizability, dielectric strength and about the Susceptibility, Permittivity, Dielectric constant with help of suitable diagrams and tables.
What is Dielectric?
Dielectrics are non-conducting substances which are the insulating materials and are bad conductor of electric current. Dielectric materials can be made to hold an electrostatic charge while dissipating minimal energy in the form of heat. Examples of dielectric are Mica, Plastics, Glass, Porcelain and Various Metal Oxides and even dry air is also example of dielectric.
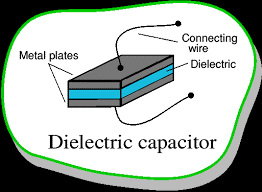
Figure (1.1) Dielectric capacitor
What is the classification of Dielectric?
Dielectrics can be classified as:
-
Polar Molecules
-
Non-Polar Molecules
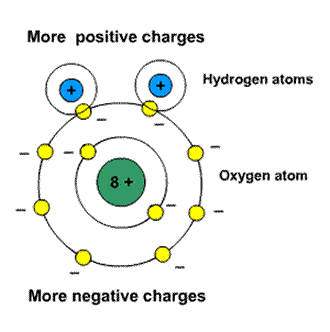
Polar Molecules: Polar Molecules are those type of dielectric in which the possibilities that the positive and negative molecules will coincide with each other is null or zero.
Fig(1.2) Coinciding polar molecules
The reason why the polar molecules do not coincide with each other is due to their shape, that is they all are asymmetric in shape.
Examples: H2O, CO2, NO2 etc.
When the electric field is not present that is if it is absent then, it causes the electric dipole moment of these molecules in random direction which is responsible for cancellation of these molecules with each other. So, the average dipole moment is zero.
Fig (1.3) Dipole moment of polar molecules
If the external electric field is present, the molecules assemble in the same direction as electric field.
Figure (1.4) Polar Molecules
Non-Polar Molecule, unlike polar molecules in non-polar molecules the center of positive charge and negative coincide, that is it is not zero. The molecule then has no permanent (or intrinsic) dipole moment.
Fig(1.5) Coinciding Non-Polar molecules
Figure (1.6) Non-polar Molecules
Induced Electric Dipole Moment
When in a non-polar molecule, all the protons are pulled in the direction as of electric field and electrons are pulled in opposite direction as of electric field, when an external electric field is applied. Due to the presence of electric field, this process continues unless the internal forces balance them. Due to this two centers of charge are created; the molecules are known as Polarized and is known as Induced Electric Dipole. The dipole moment is known as Induced Electric Dipole Moment.
Explain polarisability
Applied field is directly proportional to induced dipole moment and is independent of the temperature. The direction of induced dipole moment (x) is parallel to the direction of electric field  and for a single polar atom.
and for a single polar atom.
Polarizabilities determine the dynamical response of a bound system to external fields, and provide insight into a molecule's internal structure. In a solid, polarizability is defined as the dipole moment per unit volume of the crystal cell

where 'a' is known as Atomic Polarisability
S.I. unit and dimensions of polarisability
The S.I. unit of polarisability is m3 and it’s dimensions as same as it’s volume.
Electric Polarisation
When a dielectric slab is placedin an electric field, then the dipole moment is gained by the molecule and the dielectric is said to be polarised.
The Electric Polarization is dipole moment per unit volume of a dielectric material.
The polarization is denoted by P.
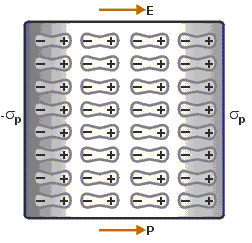 |
 |
| Figure (1.7) Polarisation | Figure(1.8) Polarisation Process |
What is Dielectric Constant?
When Dielectric slab is placed between parallel plate, the ratio of the applied electric field strength to the strength of the reduced value of electric field capacitor is called Dielectric Constant that is:

E is always less than or equal to E.
And E is net field
The larger the dielectric constant, the more charge can be stored. Completely filling the space between capacitor plates with a dielectric increases the capacitance by a factor of the dielectric constant:
C = κ Co, where Co is the capacitance with no dielectric between the plates.
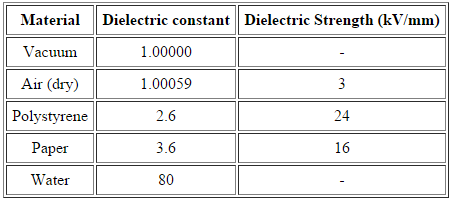
Write the Dielectric constant for materials
Dielectric constant for selected materials (~300 K except where indicated)
Dielectric Strength
For an insulating material, the dielectric strength is that ,without breaking down maximum electric field strength that it can withstand intrinsically, that is, without experiencing failure of its insulating properties is known as Dielectric Strength.
State the names and uses of material with dielectric strength
Name and Uses of materials with high dielectric strength are:
-
for electric motor windings mica is used and its stator bars.
-
The glass and porcelain are widely used for high voltage transformers and transmission line connectors
-
Naphtha or Paraffin Oil is used when it is necessary.
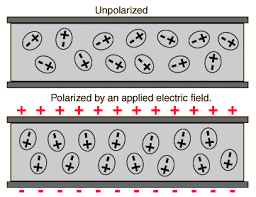
Dielectric Polarisation
When an external electric field is applied to a dielectric material, its behavior can be determined and is known as Dielectric Polarizationthat can be understood by the displacement of charges(positive and negative) when an electric field is applied
The main task of the dielectric polarization is to relate macroscopic properties to microscopic properties. Where macroscopic property can be dielectric constant to polarizability
Polarization occurs through the action of an electric field or other external factors, such as mechanical stress in the case of piezoelectric crystals (piezoelectric crystals are those solid material which accumulates electric charge within them). Dielectric Polarization can also arise spontaneously in pyroelectric crystals, particularly in ferroelectrics (Ferroelectricity is a property of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field).
Figure (1.9) Dielectric Polarisation
What is Susceptibility?
The electric susceptibility can be defined as the ratio of Polarisation P to electric field strength E,

where ϵ is the electric permittivity
In MKS, the electric susceptibility is defined as:

where ϵ0 is the permittivity of free space.
What is Permittivity?
How much a medium can be polarized in response to an applied electric field, this can determine permittivity.
Units of permittivity:
In SI units, ε0 is the permittivity of free space and has the value ε0 ≈ 1.85 × 10-12 Farads/meter.
The permittivity is given by:

Frequently Asked Questions (FAQs)
Q1. What is the difference between dielectric and electrolyte?
Sol. The difference betwwen dielectric and electrolyte are given below:
Q2. What is dielectric strength?
Sol. Place an insulator and a voltage is applied to it, it increases steadily, due to this the electrical properties will breakdown at some point. Across the electrodes, the breakdown is observed as an electrical arc which causes a sudden decrease in resistance is known as dielectric strength
Q3. State the factors on which dielectric strength depends?
Sol. The dielectric strength will depend on
-
the type and shape of the plastic and electrodes,
-
the rate with which the field is increased,
-
and the medium that surrounds the insulator.
Q4. State the applications of dielectric material?
Sol.
-
They are used in capacitance as charge storage material between the metallic plates
-
To receive microwave signals from dielectric resonator antenna.
-
Used in insulator coating of conductors and wires.
Q4. What is Polarisation density?
Sol. The vector field that expresses the density of permanent or induced electric dipole moments in a dielectric material. When in a external electric field, a electric dipole moment is gained by the molecules and the dielectric is said to be polarized. Per unit volume the electric dipole moment is induced of the dielectric material is called the Electric Polarization.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free