IIT JEE AIEEE Isomerism Course Material - Study Material
What is Isomerism?
In the study of organic chemistry we come across many cases when two or more compounds are made of equal number of like atoms. A molecular formula does not tell the nature of organic compound; sometimes several organic compounds may have same molecular formula. These compounds possess the same molecular formula but differ from each other in physical or chemical properties, are called isomers and the phenomenon is termed isomerism (Greek, isos = equal; meros = parts).
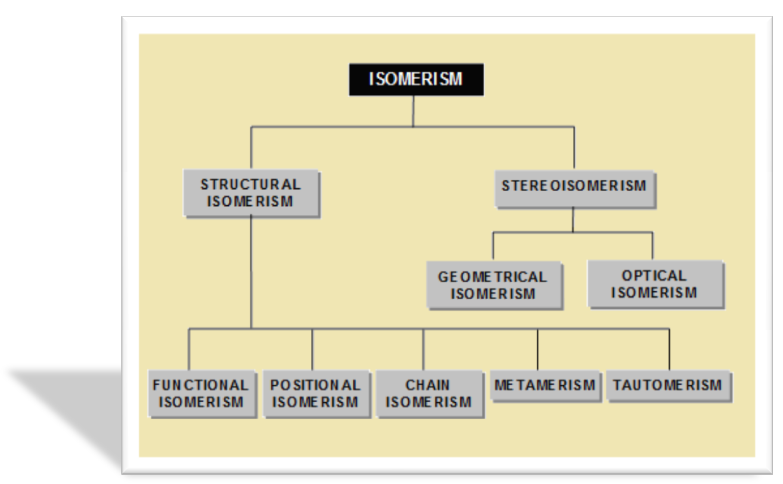
Since isomers have the same molecular formula, the difference in their properties must be due to different modes of the combination or arrangement of atoms within the molecule. Broadly speaking,there are two types of isomerism:
-
Structural Isomerism
-
Stereo Isomerism
Structural Isomerism
?When the isomerism is simply due to difference in the arrangement of atoms within the molecule without any reference to space, the phenomenon is termed structural isomerism. In other words, while they have same molecular formulas they possess different structural formulas. This type of isomerism which arises from difference in the structure of molecules, includes:
-
Chain or Nuclear Isomerism;
-
Positional Isomerism
-
Functional Isomerism
-
Metamerism and
-
Tautomerism
Stereo Isomerism
When isomerism is caused by the different arrangements of atoms or groups in space, the phenomenon is called Stereoisomerism (Greek, Stereos = occupying space). The stereoisomers have the same structural formulas but differ in the spatial arrangement of atoms or groups in the molecule. In other words, stereoisomerism is exhibited by such compounds which have identical molecular structure but different configurations.Stereoisomerism is of two types :
-
Geometrical or cis-trans isomerism
-
Optical Isomerism.
Let us explore the concepts of isomerism in organic compounds in the upcoming sub-topics.
Click here to access the Organic Chemistry Revision Notes and IIT JEE Chemistry Syllabus
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free