Collision Theory of Reaction Rate
Table of Content |
-
 A chemical reaction takes place due to collision among reactant molecules.
A chemical reaction takes place due to collision among reactant molecules. -
The number of collisions taking place per second per unit volume of the reaction mixture is known as collision frequency (Z).
-
The value of collision frequency is very high, of the order of 1025 to 1028 in case of binary collisions.
-
Every collision does not bring a chemical change.
-
The collisions that actually produce the products are effective collisions.
-
 The effective collisions which bring chemical change are few in comparison to the form a product are ineffective elastic collisions, i.e., molecules just collide anddisperse in different directions with different velocities.
The effective collisions which bring chemical change are few in comparison to the form a product are ineffective elastic collisions, i.e., molecules just collide anddisperse in different directions with different velocities. -
For a collision to be effective, the following two barriers are to be cleared.
-
Energy Barrier
-
Orientation Barrier
Refer to the following video for collision theory
Energy Barrier
The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy.
-
In the graph (Fig.8.3) 'E' corresponds to minimum or threshold energy for effective collision in a hypothetical reaction.
-
There is an energy barrier for each reaction.
-
The reacting species must be provided with sufficient energy as to cross the energy barrier.
-
The minimum amount of energy required by reactant molecules to participate in a reaction is called activation energy.
-
Activation energy = threshold energy - average kinetic energy of reacting molecules
-
Threshold energy = initial potential energy of reactant molecules + activation energy.
-
A collision between high energy molecules overcomes the forces of repulsion and brings the formation of an unstable molecule cluster, called the activated complex.
-
The life span of an activated complex is very small. Thus, the activated complex breaks either into reactants again or new substances, i.e., products.
-
The activation energy (Ea) depends upon the nature of chemical bonds undergoing rupture and is independent of enthalpies of reactants and products.
-
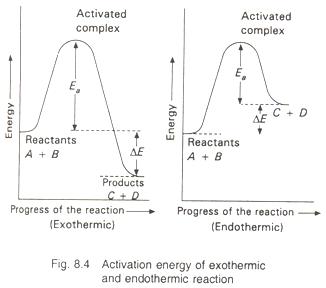
The energy changes during exothermic and endothermic reactions versus the progress of the reaction are shown in Fig.8.4.
-
Thus, every chemical reaction whether exothermic or endothermic has an energy barrier which has to be overcome before reactants can be transformed into products. If the reactant molecules have sufficient energy, they can reach the peak of the energy barrier after collision and then they can go to the right side of the slope and consequently change into products. If the activation energy for a reaction is low, the fraction of effective collisions will be large and the reaction will be fast. On the other hand, if the activation energy is high, then fraction of effective collisions will be small and the reaction will be slow. When temperature is increased, the number of active molecules increases, i.e., the number of effective collisions will increase and the rate of reaction will increase.
-
Activation energy Ea - E(activated complex) - E(ground state)
-
?H = activation energy of forward reaction - activation energy of background reactions
Note |
|
It depends on nature of reactant and is free from the effct of temperature, that is, when temperature is changed, value of activation energy is contsant. |
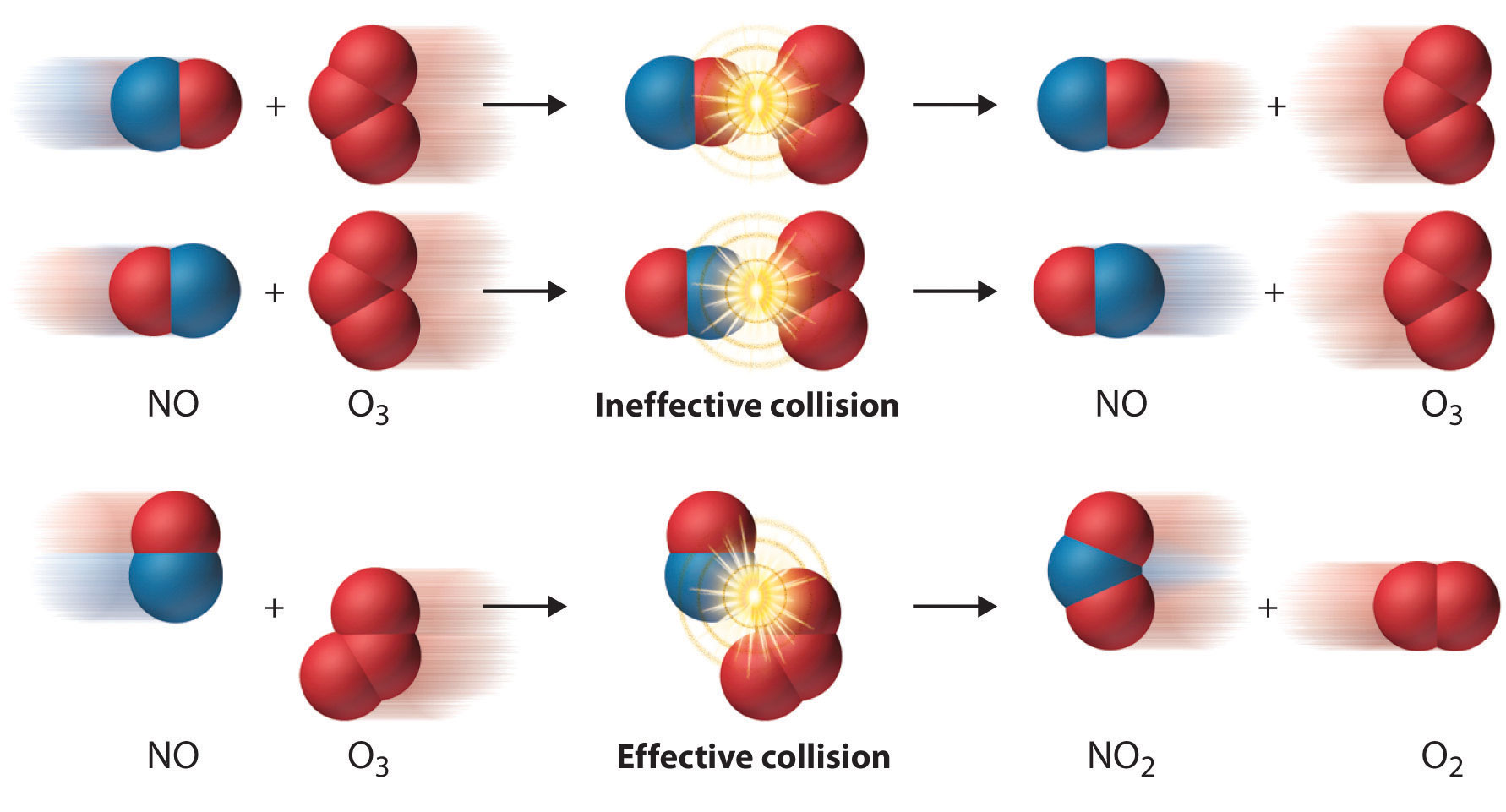
Orientation Barrier
-
Energy alone does not determine the effectiveness of the collision.
-
The reacting molecules must collide in proper manner if the reaction is to occur. This has been shown in Fig.8.5.
-
Rate of reaction is directly proportional to the number of effective collisions.
-
Rate = dx/dt = collision frequency × factor of effective collisions = z × f

Question 1:
The minimum amount of energy required by reactant molecules to participate in a reaction is called
a. reaction energy
b. activation energy
c. lattice energy
d. threshold energy
Question 2:
Activated complex is
a. reactant
b. product
c. catalyst
d. intermediate
Question 3:
The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as
a. activation energy
b. threshold energy.
c. energy barrier
d. reaction energy
Question 4:
Threshold energy = initial potential energy of reactant molecules +
a. parallel reaction
b. activation energy.
c.ionization energy

| Q.1 | Q.2 | Q.3 | Q.4 |
| b | d | b | b |
Related Resources
-
Click here to get detailed Syllabus of IIT JEE
-
Refer to the Books of Chemistry for IIT JEE
To read more, Buy study materials of Chemical Kinetics comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.

.gif)