Molecularity and Order of Reaction
Table of Content |
Molecularity of Reaction

 A chemical reaction that takes place in one and only one step i.e., all that occurs in a single step is called elementary reaction while a chemical reaction occurring in the sequence of two or more steps is called complicated reaction. The sequence of steps through which a complicated reaction takes place is called reaction – mechanism. Each step in a mechanism is an elementary step reaction.
A chemical reaction that takes place in one and only one step i.e., all that occurs in a single step is called elementary reaction while a chemical reaction occurring in the sequence of two or more steps is called complicated reaction. The sequence of steps through which a complicated reaction takes place is called reaction – mechanism. Each step in a mechanism is an elementary step reaction.
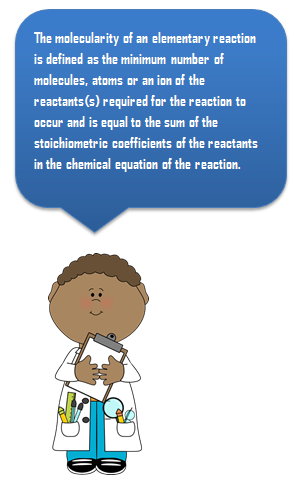
The molecularity of an elementary reaction is defined as the minimum number of molecules, atoms or ions of the reactants(s) required for the reaction to occur and is equal to the sum of the stoichiometric coefficients of the reactants in the chemical equation of the reaction.
In general, molecularity of simple reactions is equal to the sum of the number of molecules of reactants involved in the balanced stoichiometric equation.
or
The molecularity of a reaction is the number of reactant molecules taking part in a single step of the reaction.
e.g.,
Chemical Reaction |
Molecularity |
|
PCl5 → PCl3 + Cl2 |
Unimolecular |
|
2HI → H2 + I2 |
Bimolecular |
|
2SO2 + O2 → 2SO3 |
Trimolecular |
|
NO + O3 → NO2 + O2 |
Bimolecular |
|
2CO + O2 → 2CO2 |
Trimolecular |
|
2FeCl3 + SnCl2 → SnCl2 + 2FeCl2 |
Trimolecular |
The minimum number of reacting particles (molecules, atoms or ions) that come together or collide in a rate determining step to form product or products is called the molecularity of a reaction.
For example, decomposition of H2O2 takes place in the following two steps:
Decomposition of H2O2. |
||
|
Overall Reaction |
H2O2 → H2O + 1/2O2 |
|
|
Step 1: |
H2O2 → H2O + [O] |
Slow |
|
Step 2: |
[O] + [O] → O2 |
Fast |
The slowest step is rate-determining. Thus from step 1, reaction appears to be unimolecular.
There are some chemical reactions whose molecularity appears to be more than three from stoichiometric equations, e.g.
-
4HBr + O2 → 2H2O + 2Br2
-
2MNI4- + 16H+ + 5C2 O42- → 2Mn2+ + 10CO2 + 8H2O
In the first reaction molecularity seems to be '5' and in the second reaction molecularity seems to be '23'. Such reactions involve two or more steps; each step has its own molecularity not greater than three, e.g., in first reaction.
HBr + O2 → HOOBr |
|
|
Step 1: |
HOOBr + HBr → 2HOBr |
|
Step 2: |
[HOBr + HBr → H2O + Br2] |
Molecularity of each of the above steps is 2.
Reaction between Br- and H2O2 in acidic medium:
The overall reaction is
2Br- + H2O2 + 2H+ → Br2 + 2H2O
The proposed mechanism is
2Br- + H2O2 + 2H+ → Br2 + 2H2O |
||
|
Step 1: |
2Br- + H2O2 + H+ → HOBr + H2O |
Slow |
|
Step 2: |
HOBr + H+ + Br- → Br2 + H2O |
Fast |
The reaction is trimolecular
(b) Reaction between NO2 and F2:
The overall reaction is
2NO2 + F2 → 2NO2F
The proposed mechanism is
2NO2 + F2 → 2NO2F |
||
|
Step 1: |
NO2 + F2 → NO2 + F |
Slow |
|
Step 2: |
NO2 + F → NO2F |
Fast |
The reaction is bimolecular.
Reactions of higher molecularity (molecularity > 3) are rare. This is because a reaction takes place by collision between reactant molecules and as number of reactant molecules i.e. molecularity increases the chance of their coming together and colliding simultaneously decreases.
Refer to the following video for order and molecularity of reaction
Order of Reaction
The mathematical expression showing the dependence of rate on the concentration(s) of reactant(s) is known as rate-law or rate-expression of the reaction and sum of the indices (powers) of the concentration terms appearing in the rate law as observed experimentally is called order of reaction. To understand what is order of reaction, consider the reaction
2NO(g) + 2H2(g) → N2(g) + 2H2O (g)
Kinetic experiment carried out at 1100 K upon this reaction has shown following rate data.
Expt. No. |
[NO] (mole dm–3) |
[H2] (mole dm–3) |
Rate (mole dm–3s–1) |
|
1. |
5 |
2.5 |
3 |
|
2. |
1.0 |
2.5 |
1.2 |
|
3. |
1.0 |
5.0 |
2.4 |
 From the Expt. No.1 and 2, it is evident that rate increases 4 fold when concentration of NO is doubled keeping the concentration of H2 constant i.e.
From the Expt. No.1 and 2, it is evident that rate increases 4 fold when concentration of NO is doubled keeping the concentration of H2 constant i.e.
-
Rate
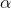 [NO]2 when [H2] is constant again from Expt. No.2 and 3, it is evident that when concentration of H2 is doubled keeping the concentration of NO constant, the rate is just doubled i.e.
[NO]2 when [H2] is constant again from Expt. No.2 and 3, it is evident that when concentration of H2 is doubled keeping the concentration of NO constant, the rate is just doubled i.e. -
Rate
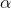 [H2] when [NO] is constant
[H2] when [NO] is constant -
From Expt. (1) and Expt. (3), the rate increases 8-fold when concentrations of both NO and H2 are doubled simultaneously i.e.
-
Rate
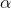 [NO]2 [H2]
[NO]2 [H2] - This is the rate-law of reaction as observed experimentally. In the rate law, the power of nitric oxide concentration is 2 while that of hydrogen concentration is 1. So, order of reaction w.r.t. NO is 2 and that w.r.t. H2 is 1 and overall order is 2 + 1 i.e. 3.
Note that the experimental rate law is not consistent with the stoichiometric coefficient of H2 in the chemical equation for the reaction. This fact immediately suggests that the reaction is complicated and it does not occur in single step as written. In order to explain the rate law, following mechanism has been proposed.
-
NO + NO
 N2O2 (fast and reversible)
N2O2 (fast and reversible) -
N2O2 + H2 → N2O + H2O (slow)
-
N2O + H2 → N2 + H2O (fast)
Let us see how this mechanism corresponds to the rate law as found experimentally and mentioned above.
-
The step II being the slowest step is the rate-determining step. Thus, rate of overall reaction or rate of formation of N2, will be equal to the rate of step II or rate of formation of H2O. So, we have according to Law of Mass Action.
-
Rate of overall reaction = Rate of step II = k [N2O2] [H2]
-
Where k = rate constant of step II.
-
N2O2 being intermediate for the overall reaction, its concentration has to be evaluated in terms of the concentration(s) of reactant(s) and this can be done by applying Law of Mass Action upon the equilibrium of Step I. Thus,

where KC = equilibrium constant of Step II. Putting this value of concentration of N2O2 in the above rate expression, we get
Rate reaction = k×Kc× [NO]2 [H2]
or Rate of reaction = k1[NO]2 [H2]
Rate of reaction 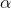 [NO]2 [H2]
[NO]2 [H2]
Where k×Kc = k1 = another constant, rate constant of overall reaction.
Note that from the knowledge of any two out of k, Kc and k1, the rest one may be calculated.
We are again turning to our topic “order”. In general, if rate law of a reaction represented by the equation.
aA + bB → Products
is experimentally found to be as follows:
Rate 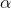 [A]m [B]n
[A]m [B]n
Then
order w.r.t. A = m
order w.r.t. B = n
overall order = m + n
It is to be noted that ‘m’ may or may not be equal to ‘a’ and similarly. ‘n’ may or may not be equal to ‘b’. m and n are experimental quantities and their values which really depend on the reaction-mechanism and experimental condition, may not be predicted by just seeing the chemical equation of the reaction. Reactions with some kind of chemical equations may differ in this rate laws and hence order. An example of this is as follows.
Reactions |
Rate Law |
Order |
|
2N2O5 → 4NO2 + O2 |
Rate |
1 |
|
2NO2 → N2O2 +O2 |
Rate |
2 |
Order of reaction may also be defined as follows.
Number of molecules of the reactant(s) whose concentration changes during the chemical change is called order of reaction.
For example, the reaction
CH3COOC2H5 + H2O  CH3COOH + C2H5OH
CH3COOH + C2H5OH
is a bimolecular reaction but its order is ‘one’. This is because during the reaction only the concentration of ester decreases with time.
The concentration of water in the reaction mixture (usually a dilute aqueous solution of ester mixed with dilute aqueous acid) being in large excess as compared to ester, does not decrease appreciably or measurably during the reaction.
The first order behaviour of this reaction can be derived in the following way.
Applying Law of Mass Action upon the above reaction.
Rate 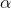 [CH3COOC2H5] [H2O]
[CH3COOC2H5] [H2O]
or Rate = k[CH3COOC2H5] [H2O]
Where k is the rate constant of the above bimolecular reaction.
Since concentration of water remains practically constant. So,
K[H2O] = k1 = another constant or observed rate constant of the reaction.
So,
Rate = k1 [CH3COOC2H5]
This is first-order kinetics i.e. order in respect of ester is ‘1’ and that in respect of water is ‘zero’.
The reaction is an example of pseudounimolecular (or pseudo first order).
Thus, a second order reaction conforms to the first order if out of the reactants one is present in large excess and the reaction is called pseudounimolecular.
|
Suppose we have a reaction: 2A + B → Products With rate law Rate µ [A]2 [B] (order = 2 + 1 = 3) It B is taken in large excess as compared to A, their reaction will obey the kinetics. Rate So, Order w.r.t. A = 2 Order w.r.t. B = 0 Overall order = 2 + 0 = 2 If A is taken in large excess as compound to B then reaction will obey the kinetics. Rate So, Order w.r.t. A = 0 Order w.r.t. B = 1 Overall order = 0 + 1 = 1 If both A and B are taken in large excess, can you say what will be the order? Some of you may tell that order will be zero. This is absolutely wrong. When both A and B are in large excess, then there will be appreciable damage in the concentrations of both of them and hence order will be ‘3’. Reactions are classified on the basis of order as an, first, second, third order etc. according as their order equal to 0, 1, 2and 3 etc. respectively |
Some typical linear plots for the reactions of different orders: |
 |
Plots rate vs concentrations [Rate = k(conc.)n]
From the study of the kinetics of many simple reactions, it is observed that for a large number of reactions, the molecularity and order are the same. Some examples are given below to justify this point.
-
Dissociation of N2O5.
N2O5 → N2O4 + O2
Order = 1, Molecularity = 1
-
Dissociation of H2O2.
H2O2 → H2O + 1/2O2
Order = 1, Molecularity = 1
-
Dissociation of HI,
2HI → H2 + I2
-
Formation of NO2.
2NO + O2 → 2NO2
Order = 3, Moelcularity =3
Pseudo- Order Reactions
Reactions whose actual order is different from that expected using rate law expression are called pseudo-order reactions; e.g.,
RCl + H2O → ROH + HCl
Expected rate law:
Rate = k[RCl] [H2O] Expected order = 1+1 =2
Actual rate law:
Rate = k'[RCl]; Actual order = 1
Water is taken in excess; therefore, its concentration may be taken constant. The reaction is, therefore, pseudo first order. Similarly, the acid catalysed hydrolysis of ester, viz.,
RCOR' + H2O ↔ RCOOH + R'OH
follow first order kinetics:
Rate = k[RCOOR']
It is also a pseudo-first order reaction.
The Main differences between Molecularity and Order of Reaction
Moleculariy |
Order of Reaction |
|
It is the total number of reacting species (molecules, atoms or ions) which bring the chemical change. |
It is the sum of powers of molar concentration of the reacting species in the rate equation of the reaction. |
|
It is always a whole number. |
It may be a whole number, zero, fractional, |
|
It is a theoretical concept. |
It is experimentally determined. |
|
It is meaningful only for simple reactions or individual steps of a complex reaction. It is meaningless for overall complex reaction. |
It is meant for the reaction and not for its individual steps |

Question 1: Which of the following reaction is bimolecular one?
a. PCl5 → PCl3 + Cl2
b. H2O2 → H2O + 1/2O2
c. N2O5 → N2O4 + O2
d. N2O + H2 → N2 + H2O
Question 2: Which of the following statements about molecularity of any reaction is correct?
a. It is experimentally determined
b. It is meant for the reaction and not for its individual steps
c. It may or may not be whole number.
d. It can never me zero
Question 3: Rate of a reaction depends on
a. slow step
b. fast step
c. overall reaction
d. both slow and fast step
Question 4:
Molecularity of the reaction 2HI → H2 + I2 is?
a.1
b. 2
d.3/2

| Q.1 | Q.2 | Q.3 | Q.4 |
| d | d | a | b |
Related Resources
-
Click here for the detailed Syllabus of IIT JEE
-
Look here for Useful Books of Chemistry for IIT JEE
-
You can also refer to Introduction to chemical kinetics
To read more, Buy study materials of Chemical Kinetics comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.

 10–3
10–3