Parallel and Sequential Reactions
Table of Content |
Parallel or Competing Reaction
 The reactions in which a substance reacts or decomposes in more than one way are called parallel or side reactions.
The reactions in which a substance reacts or decomposes in more than one way are called parallel or side reactions.
If we assume that both of them are first order, we get.
![-\frac{d[A]}{dt} = (k_1 +k_2) [A] =k_{av}[A]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex-_fracdadtk_1k_2ak_ava.jpg)
k1 = fractional yield of B × kav
k2 = fractional yield of C × kav
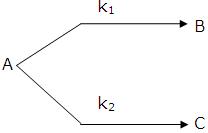
If k1 > k2 then
A → B main and
Let after a definite interval x mol/litre of B and y mol/litre of C are formed.
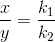
i.e
![\frac{\frac{d[B]}{dt}}{\frac{d[C]}{dt}} =\frac{k_1}{k_2}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_frac_fracdbdt_fracdcdt_frack_1k_2.jpg)
This means that irrespective of how much time is elapsed, the ratio of concentration of B to that of C from the start (assuming no B and C in the beginning ) is a constant equal to k1/k2.
Refer to the following video for parallel reactions:
Example of Parallel Reactions |
|
|
Consecutive or Sequential Reactions
This reaction is defined as that reaction which proceeds from reactants to final products through one or more intermediate stages. The overall reaction is a result of several successive or consecutive steps.
A → B → C and so on
Example of Sequential Reactions |
(CH2)2O CH3CHO
(CH3)2CO CH2 =C=O |
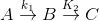
![-\frac{d[A]}{dt} = k_1[A]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex-_fracdadtk_1a.jpg) …....(i)
…....(i)
![\frac{d[B]}{dt} = k_1[A]-K_2[B]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_fracdbdtk_1a-k_2b.jpg) …......(ii)
…......(ii)
![\frac{d[C]}{dt} = k_2[B]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_fracdcdtk_2b.jpg) ….......(iii)
….......(iii)
Integrating equation (i), we get
![[A]-[A]_oe^{-k_1t}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexa-a_oe-k_1t.jpg)
Now we shall integrate equation (ii) and find the concentration of B related to time t.

Integration of the above equation is not possible as we are not able to separate the two variables, [B] and t. Therefore we multiply equation (4) by an integrating factor  , on both the sides of the equation.
, on both the sides of the equation.

Integrating with in the limits 0 to t.

Now in order to find [C], substitute equation (vi) in equation (iii), we get

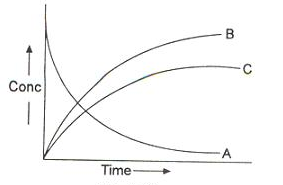
Bmax and tmax:
We can also attempt to find the time when [B] becomes maximum. For this we differentiate equation (vi) and find d[B]/dt & equate it to zero.

Substituting equation (vii) in equation (vi)


Question 1:
Parallel reactions take place in
a. more than one way
b. more than one step
c. in one way but more than one step
d. in one step but more than one way
Question 2:
Sequential reactions have
a. more than one way to proceed toward product
b. more than one step
c. no intermediate
d. catalyst
Question 3:
Which one of the following statements is correct?
a. parallel reactions are also known as consecutive reactions
b. Competingreactions have more than one step
c. Sequential reactions are also called consecutive reactions
d. Consecutive reactions do not have intermediates.
Question 4:
Decomposition of ethylene oxide is
a. parallel reaction
b. competing reaction
c.both parallel as well as competing reaction

| Q.1 | Q.2 | Q.3 | Q.4 |
| a | b | c | d |
Related Resources
-
Click here to get detailed Syllabus of IIT JEE
-
Refer to the Books of Chemistry for IIT JEE
-
You can also refer Zero Order Reaction
To read more, Buy study materials of Chemical Kinetics comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free



 CH3CHO
CH3CHO CO + CH4
CO + CH4