Properties of Alpha, Beta and Gamma Rays
Table of Content |
 When an atom undergoes radioactivity, it emits particles like alpha, beta and gamma rays. Radioactivity basically occurs because the unstable atom tries to attain stability. Hence, when they are unstable, they eventually decay by emitting a particle transforming the nucleus into another nucleus, or into a lower energy state. This chain of decays continues till the nucleus attains the stage of stability.
When an atom undergoes radioactivity, it emits particles like alpha, beta and gamma rays. Radioactivity basically occurs because the unstable atom tries to attain stability. Hence, when they are unstable, they eventually decay by emitting a particle transforming the nucleus into another nucleus, or into a lower energy state. This chain of decays continues till the nucleus attains the stage of stability.
There are basically three types of radiations that are emitted by radioactive particles. These three are called the alpha, beta and gamma rays. All these radiations are released from the nucleus of the atom. Though all three cause some ionization and have some penetration power, but their behavior differs from the others. We discuss the properties of alpha, beta and gamma rays in detail one by one:
Properties of Alpha Rays
Alpha rays or alpha particles are the positively charged particles. A highly energetic helium nucleus which contains two protons and two neutrons is called the alpha-particle.
Alpha particles have the least penetration power but the greatest ionization power. They cannot penetrate the skin but this does not mean that they are not dangerous. Since they have a great ionization power, so if they get into the body they can cause serious damage. They have the ability of ionizing numerous atoms I a short distance. It is due to this reason that the radioactive substance that releases alpha particles needs to be handled with rubber gloves. It should not be inhaled, eaten or allowed near open cuts.
Beta Rays
 Beta particles are highly energetic electrons which are released from inside of a nucleus. They are negatively charged and have a negligible mass. On the emission of a beta particle, a neutron in the nucleus divides into a proton and an electron. The beta particle is thus the electron that is rejected from the nucleus at high speed. Beta particles have a greater penetration power than the alpha particles and can easily travel through the skin. Though beta particles have less ionization power than the alpha particles but still they are dangerous and so their contact with the body must be avoided.
Beta particles are highly energetic electrons which are released from inside of a nucleus. They are negatively charged and have a negligible mass. On the emission of a beta particle, a neutron in the nucleus divides into a proton and an electron. The beta particle is thus the electron that is rejected from the nucleus at high speed. Beta particles have a greater penetration power than the alpha particles and can easily travel through the skin. Though beta particles have less ionization power than the alpha particles but still they are dangerous and so their contact with the body must be avoided.
Gamma Rays
The waves from the high frequency end of the electromagnetic spectrum which do not have any mass are called the gamma rays. They have greatest power of penetration. They are the least ionizing but most penetrating and it is extremely difficult to stop them from entering the body. These rays carry huge amount of energy and can even travel through thin lead and thick concrete.
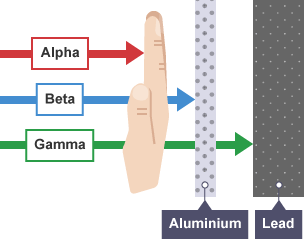
Most of the properties of alpha, beta and gamma particles have been already discussed. Given below is the table of characteristics of alpha, beta and gamma radiations which also compares the charges and masses of the three rays and the above figure shows the penetration power of alpha, beta and gamma rays.
|
Property |
α ray |
β ray |
|
|
Nature |
Positive charged particles, 2He4nucleus |
Negatively charged particles (electrons). |
Uncharged γ~0.01a, electromagnetic radiation |
|
Charge |
+2e |
–e |
0 |
|
Mass |
6.6466 × 10–27 kg |
9.109 × 10–31 kg |
0 |
|
Range |
~10 cm in air, can be stopped by 1mm of A l |
Upto a few m in can be stopped~cm of A l |
Several m in air stopped by~cm of Pb |
|
Natural Sources |
By natural radioisotopes e.g.92U236 |
By radioisotopes e.g.29Co68 |
Excited nuclei formed as a result of α, β decay |
Watch this Video for more reference
|
|
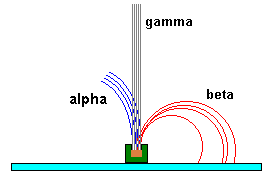
Alpha, beta and gamma radiation can be detected by using magnetic field. Alpha and beta particles have contrary charges-they undergo deflection in opposite direction, whereas gamma rays do not transfer any charge-they do not undergo deflection.The displacement laws of radioactivity governed by Rutherford, which are listed below:-
(a) During a α decay, the daughter element was always two position below the parent in the periodic table; the mass number of the daughter nucleus was 4 units smaller than that of the parent nucleus.
(b) During β-decay, the daughter nucleus had the same mass number as the parent while its atomic number was greater than that of the parent by 1 (smaller by 1 unit in β+ decay).
(c) g-emission did not result in change of atomic number or mass number. Radioactivity is observed to be a random process, it cannot be predicted when a particular nucleus will decay. One can only discuss the probability of its decay.


Question 1 (JEE Main)
When a b- particle is emitted from a nucleus, the neutron-proton ratio
(a) is decreased (b) is increased
(c) remains the same (d) may increase or decrease
Question 2 (JEE Advanced)
A g – ray photon is emitted
(a) after ionization of an atom
(b) due to conversion of a neutron into a proton in the nucleus
(c) after de-excitation of a nucleus
(d) due to conversion of a proton into a neutron in the nucleus
Question 3
If a nucleus ZXA emits one a-particle and one b(negative b) particle in succession, then the daughter nucleus will have which of the following configurations?
(a) A-4 nucleons (b) A-3 nucleons
(c) A-Z-3 neutrons (d) Z-2 protons
Question 4
In which of the following decays, the element does not change?
(a) a – decay (b) b + – decay


| Q.1 | Q.2 | Q.3 | Q.4 |
|
a |
c |
a & c |
d |
Related Resources
-
You might like to refer Radioactivity.
-
For getting an idea of the type of questions asked, refer the Previous Year Question Papers.
-
Click here to refer the most Useful Books of Physics.
-
To get answer to any question related to properties of alpha,beta and gamma rays click here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free