Balancing Redox Reactions
Table of Content |
Oxidation Number / State Method For Balancing Redox Reactions
This method is based on the principle that the number of electrons lost in oxidation must be equal to the number of electrons gained in reduction. The steps to be followed are :
-
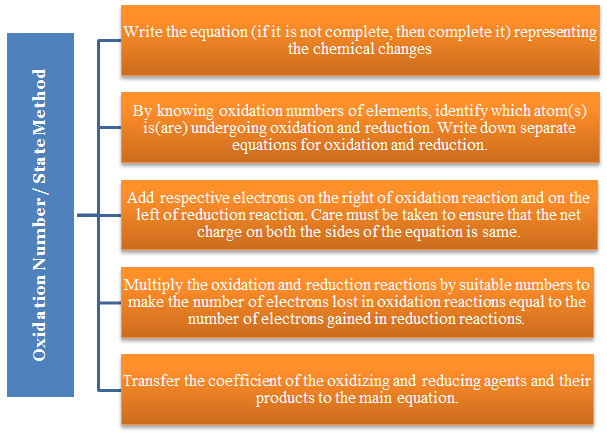
Write the equation (if it is not complete, then complete it) representing the chemical changes.
-
By knowing oxidation numbers of elements, identify which atom(s) is(are) undergoing oxidation and reduction. Write down separate equations for oxidation and reduction.
-
Add respective electrons on the right of oxidation reaction and on the left of reduction reaction. Care must be taken to ensure that the net charge on both the sides of the equation is same.
-
Multiply the oxidation and reduction reactions by suitable numbers to make the number of electrons lost in oxidation reactions equal to the number of electrons gained in reduction reactions.
-
Transfer the coefficient of the oxidizing and reducing agents and their products to the main equation.
-
By inspection, arrive at the co-efficients of the species not undergoing oxidation or reduction.
Half-Reaction or Ion-Electron Method For Balancing Redox Reactions
 This method involves the following steps :
This method involves the following steps :
-
Divide the complete equation into two half reactions, one representing oxidation and the other reduction.
-
Balance the atoms in each half reaction separately according to the following steps:
-
First of all balance the atoms other than H and O.
-
In a reaction taking place in acidic or neutral medium, oxygen atoms are balanced by adding molecules of water to the side deficient in oxygen atoms while hydrogen atoms are balanced by adding H+ ions to the other side deficient in hydrogen atoms. On the other hand, in alkaline medium (OH-), every excess of oxygen atom on one side is balanced by adding one H2O to the same side and 2OH- to the other side. In case hydrogen is still unbalanced, then balance by adding one OH-, for every excess of H atom on the same side as the excess and one H2O on the other side.
-
Equalize the charge on both sides by adding a suitable number of electrons to the side deficient in negative charge.
-
Multiply the two half reactions by suitable integers so that the total number of electrons gained in one half reaction is equal to the number of electrons lost in the other half reaction.
-
Watch this Video for more reference
-
Add the two balanced half equations and cancel any term common to both sides.

Question 1: Which of the following equations represents a balanced redox reaction?
a. KMnO4 + H2SO4 + FeSO4 → K2SO4 + MnSO4 + Fe2(SO4)3 + H2O
b. 2KMnO4 + 10 FeSO4 + 8H2SO4 → K2SO4 + 2Mn(SO4) + 5Fe2(SO4)3 + 8H2O
c. Cr2O7 2-(g) + SO2(aq) → Cr3+ + SO42-(aq)
d. Al + NO3- → Al(OH)4- +NH3
Question 2: Ion-electron method for balancing Redox reactions is also known as
a. Oxidation number method
b. State method
c. Half reaction method
d. Half life method
Question 3: In a reaction taking place in acidic or neutral medium, oxygen atoms are balanced by adding
a. H2O & H+
b. H2O & OH-
c. H+ & OH-
d. H2O only
Question 4: What is the number of electrons being transferred in the redox reaction 2KMnO4 + 10 FeSO4 + 8H2SO4 → K2SO4 + 2Mn(SO4) + 5Fe2(SO4)3 + 8H2O?
a. Zero
b. One
c. Five

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
b |
c |
a |
d |
Related Resources
-
Click here to know the syllabus of chemistry for IIT JEE
-
Have a look at syllabus of IIT JEE
-
You can refer to oxidation number
To read more, Buy study materials of Redox Reactions comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More


 2; 2Fe+2 → Fe2+3 + 2e- (2)
2; 2Fe+2 → Fe2+3 + 2e- (2)