Alkaline Earth Metals
Table of Content |
|
|
 The group 2 of the periodic table consists of six metallic elements. They are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). The name alkaline earth metals was given to magnesium, calcium, barium & strontium since their oxides were alkaline in nature and these oxide remained unaffected by heat or fire and existed in earth.
The group 2 of the periodic table consists of six metallic elements. They are Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). The name alkaline earth metals was given to magnesium, calcium, barium & strontium since their oxides were alkaline in nature and these oxide remained unaffected by heat or fire and existed in earth.
So, group 2 metals are called alkali earth metals because their hydroxides are strong alkali (just like those of alkali metals) plus these all are found in earth crust.
Occurrence of Alkali Earth Metals
Like alkali metals, alkaline earth metals are also highly reactive and hence do not occur in the free state but are likely distributed in nature in the combined state as silicates, carbonates, sulphates and phosphates.
|
Elements |
Abundance |
Main Minerals |
Uses |
|
Beryllium |
2.8 to 10-3% |
First detected in 1798 in the gemstone beryl and emerald (Be3Al2Si6O1) |
Used in corrosion resistant alloys. |
|
Magnesium |
2.33%, 7th most abundant element in earth’s crust |
Pure Mg first prepared in 1800, named after the magnesia district in Thessaly Greece where large deposits of the mineral are found |
When alloyed with Al, Mg is widely used as structural materials because of its high strength, low density and ease in machining. |
|
Calcium |
4.15%, 5thmost abundant element in earth’s crust. |
CaCO3.2H2O obtained in pure form in 1808, calcium is derived from latin word calx, meaning “lime” |
As an alloying agent for hardness in aluminium compounds. Calcium is the primary constituent of teeth and bones. |
|
Strontium |
0.038% |
Discovered in 1787 and named after the small town of strontion (Scotland) |
SrCO3 is used for the manufacture of glass for colour TV picture tubes. |
|
Barium |
0.042% |
Found in minerals witherite (BaCO3) and barite (BaSO4) after which it is named. |
BaSO4 is used in medicine as a contrast medium for stomach and intestine X – rays |
|
Radium |
Traces |
Isolated as chloride in 1898 from the mineral pitchblende |
Used in cancer radiotheraphy |
Group IIA (Alkaline earth metals) and groups IIB (Zn, Cd, Hg) Mg acts as a bridge element between IIA and IIB.
Electronic Configuration
The general electronic configuration of alkaline earth metals is ns2.
|
Elements |
Electronic Configuration |
|
Be |
1s22s2 |
|
Mg |
1s22s2sp63s2 |
|
Ca |
|
|
Sr |
[Kr]5s2 |
|
Ba |
|
|
Ra |
[Rn]7s2 |
Physical Properties of Group II elements
Atomic and Ionic Radii
The atomic radii as well as ionic radii of the members of the family are smaller than the corresponding members of alkali metals.
Ionization Energy
The alkaline earth metals owing to their large size of atoms have fairly low values of ionization energies as compared to the p – block elements. However with in the group, the ionization energy decreases as the atomic number increases. It is because of increase in atomic size due to addition of new shells and increase in the magnitude of screening effect of the electrons in inner shells. Because their (IE)1 is larger than that of their alkali metal neighbours, the group IIA metals trend to the some what less reactive than alkali metals.
The general reactivity trend is Ba > Sr > Ca > Mg > Be.
Oxidation State
The alkaline earth metal have two electrons in their valence shell and by losing these electrons, these atoms acquire the stable noble gas configuration. Thus, unlike alkali metals, the alkaline earth metals exhibit +2 oxidation state in their compounds.
Density of Alkali Earth Metals
Atomic weight increases from Be to Ba in a group and volume also increases, but increase in atomic weight is more as compare to atomic volume. Therefore the density increases from Be to Ba.
Exception: Density of Mg is more as compare to that of Ca.
Melting and Boiling Points
The alkaline earth metals have higher melting and boiling points as compared to those of alkali metals which is attributed to their small size and more close packed crystal lattice as compared to alkali metals and presence of two valence electrons.
Heat of Hydration
The heats of hydration of M2+ decreases with an increase in their ionic size and their values are greater than that of alkali metal ions.
Alkaline earth metal ions, because of their larger charge to size ratio, exert a much stronger electrostatic attraction on the oxygen of water molecule surrounding them.
Since the alkaline earth metals (except Be) tend to lose their valence electrons readily, they act as strong reducing agents as indicated by E0 red values. The particularly less negative value for Be arises from the large hydration energy associated with the small size of Be2+ and the relatively large value of heat of sublimation.
Solubility
Basic nature of oxides increases down the group but solubilities of sulphates and carbonates decrease as ionic size increases.
The solubility of most salts decreases with increased atomic weight, though usual trend is reversed with fluorides and hydroxides in this group.
|
Property |
Elements |
||||||
|
Be |
Mg |
Ca |
Sr |
Ba |
Ra |
||
|
Atomic number |
4 |
12 |
20 |
38 |
56 |
88 |
|
|
Atomic mass |
9.01 |
24.31 |
40.08 |
87.62 |
137.33 |
226.03 |
|
|
Metallic radius/pm |
112 |
160 |
197 |
215 |
222 |
- |
|
|
Ionic radius/pm |
51 |
72 |
100 |
118 |
135 |
148 |
|
|
Ionization enthalpy (kJ mol-1) |
I |
899 |
737 |
590 |
549 |
503 |
509 |
|
II |
1757 |
1450 |
1146 |
1064 |
965 |
979 |
|
|
Enthalpy of hydration of M2+ ions (kJ mol-1) |
-2494 |
-1921 |
-1577 |
-1443 |
-1305 |
- |
|
|
Electronegativity (Pauling Scale) |
1.57 |
1.31 |
1.00 |
0.95 |
0.89 |
0.9 |
|
|
Density/g mol- at 298 K |
1.85 |
1.74 |
1.55 |
2.63 |
3.62 |
5.5 |
|
|
Melting Point/K |
1562 |
924 |
1124 |
1062 |
1002 |
973
|
|
|
Boiling point /K |
2745 |
1363 |
1767 |
2078 |
(1973) (uncertain) |
||
|
E°(V) at 298 K for M2+(aq) + 2e- → M(s) |
-1.97 |
-2.37 |
-2.87
|
-2.89 |
-2.90 |
-2.92 |
|
|
Occurrence in Lithosphere |
2* |
2.76** |
4.6** |
384* |
390* |
10-10** |
|
Reactivity and Electrode potential
All the alkaline earth metals are highly reactive elements since they have a strong tendency to lose the two valences s-electrons to form the corresponding dipositive ions having inert gas configuration. The high reactivity arises due to their low ionization energies and high negative values of their standard electrode potentials. Further, the chemical reactivity of alkaline earth metals increase on moving down the group because the I.E. decreases and electrode potentials become more and more negative with increasing atomic number from Be to Ra. Thus, beryllium is the least reactive while Ba (or Ra) is the most reactive element. Further since the ionization energies of alkaline earth metals are higher and their electrode potential is less negative than the corresponding alkali metals. They are less reactive than corresponding alkali metals.
Reducing Character
The alkaline earth metals are weaker reducing agents than the alkali metals. Like alkali metals, their reducing character also increases down the group. This is due to the reason that the alkaline earth metals have greater tendency to lose electrons so, they act as reducing agent but since their I.E. are higher and their electrode potentials are less negative than the corresponding alkali metals, therefore alkaline earth metals are weaker reducing agents than alkali metals. The sulphates are stable to heat whereas the carbonates decompose to give MO and CO2, the temperature of decomposition increasing from Mg to Ba. BeCO3 is kept in the atmosphere of CO2 to prevent its decomposition.
Ba and Mg do not impart any colour to the fame i.e. they do not give flame test. This is due to their very small size. Ca, Sr and Ba impart brick red, Blood red and Apple green colours respectively to the flame.
Refer to the following video for flame test of alkali metals
Solved Examples |
|
Question 1: The alkaline earth metals shows +2 oxidation state i.e. they always form divalent cations (M2+). Explain. Solution: If ionization energy were the only factor involved, than group II elements should have formed monovalent ions i.e. Mg+, Ca+ etc rather than Mg2+, Ca+2 etc.
The heat of hydration (hydration energy) of alkaline earth metals are approximately four times higher than alkali metals of comparable size. e.g. ΔHhyd for Na+ (size 102 pm) = -397 KJmol-1 ΔHhyd for Ca+2 (size 100 pm) = -1650 KJmol-1 Larger hydration energy is due to the fact that the alkaline earth metals ions, because of their much larger charge to size ratio, exert a much stronger electrostatic attraction on the oxygen of water molecule. _____________________________ Question 2: The 2nd ionization energies of the elements of group I are higher than those of the elements of group II. Explain. The 2nd electron in case of alkali metal is to be removed form a cation (unipostive ion) which has already acquired a noble gas configuration whereas in case of alkaline earth metals, the second electron is to be removes fro a cation which is yet to acquire the stable noble gas configuration therefore, removal of 2nd electron in case of alkaline earth metals requires much less energy than that in case of alkali metals. There is sharp increase in third ionization energy due to stable inert gas configuration of m+2 ions. This explains the upper limit of +2 oxidation state for the elements. |
Difference between Alkaline Earth Metals and Alkali Metals
Both alkaline earth metals and alkali metals are s – block elements as the last electron enters the ns – orbital. They resemble with each other in some respects but still there are certain dissimilarities in their properties on account of different number of electrons in the valency shell, smaller atomic radii, high ionization potential, higher electro negativity etc.
|
|
Properties |
Alkaline earth metals |
Alkali metals |
|
1. |
Electronic configuration |
Two electrons are present in the valency shall. The configuration is ns2 (bivalent) |
One electron is present in the valency shell. The configuration is ns1 (monovalent) more electropositive |
|
2. |
Valency |
Bivalent |
Monovalent |
|
3. |
Electropositive nature |
Less electropositive |
More electropositive |
|
4. |
Hydroxides |
Weak bases, less soluble and decompose on heating. |
Strong bases, highly soluble and stable towards heat. |
|
5. |
Bicarbonates |
These are not known in free state. Exist only in solution. |
These are known in solid state. |
|
6. |
Carbonates |
Insoluble in water. Decompose on heating. |
Soluble in water. Do not decompose on heating (LiCO3 is an exception) |
|
7. |
Action of nitrogen |
Directly combine with nitrogen and form nitrides |
Do not directly combine with nitrogen except lithium |
|
8. |
Action of carbon |
Directly combine with carbon and form carbides |
Do not directly combine with carbon |
|
9. |
Nitrates |
Decompose on heating evolving a mixture of NO2 and oxygen |
Decompose on heating evolving only oxygen |
|
10. |
Solubility of salts |
Sulphates, phosphates fluorides, chromates, oxalates etc are insoluble in water |
Sulphates, phosphates, fluorides, chromates, oxides etc are soluble in water. |
|
11. |
Physical properties |
Comparatively harder. High melting points. Diamagnetic. |
Soft, low melting points paramagnetic. |
|
12. |
Hydration of compounds |
The compounds are extensively hydrated. MgCl2.6H2O, CaCl2.6H2O, BaCl2.2H2O are hydrated chlorides. |
The compounds are less hydrated. NaCl, KCl, RbCl form non – hydrated chlorides |
|
13. |
Reducing power |
Weaker as ionization potential values are high and oxidation potential values are low. |
Stronger as ionization potential values are low and oxidation potential values are high. |
Refer to the following video for alkali metals and alkali earth metals
Chemical Properties of Alkali Earth Metals
Reaction With Hydrogen – (Formation of Hydrides)
All the alkaline earth metals except be combine with hydrogen directly on heating to form metal hydrides of formula MH2.
M + H2  MH2
MH2
The hydride of beryllium can also be obtained by the reduction of BeCl2 with LiAlH4
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
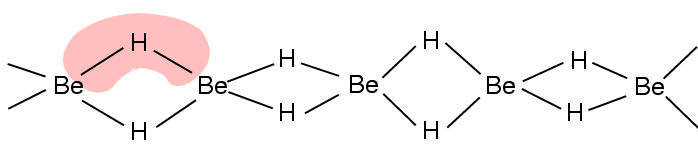
Both BeH2 and MgH2 are covalent compounds having polymeric structures in which H – atoms between beryllium atoms are held together by three
centre – two electron (3C - 2e) bonds as shown below:
The hydrides of other elements of this group i.e. CaH2, SrH2 and BaH2 are ionic and contain the H- ions.
All the hydrides of alkaline earth metals reacts with water liberating H2 gas and thus act as reducing agents.
MH2 + 2H2O → M (OH)2 + 2H2
CaH2 is called Hydrolith and is used for production of H2 by action of water on it.
Reaction With Carbon – (Formation of Carbides)
When BeO is heated with carbon at 2175 – 2275 K a brick red coloured carbide of the formula Be2C is formed
2BeO + 2C  Be2C + 2CO.
Be2C + 2CO.
It is a covalent compound and react water forming methane.
Be2C + 4H2O → 2Be (OH)2 + CH4
The rest of the alkaline earth metals (Mg, Ca, Sr & Ba) form carbides of the general formula, MC2 either when the metal is heated with carbon in an electric furnace or when their oxides are heated with carbon.
Ca + 2C  CaC2
CaC2
CaO + 3C  CaC2 + CO
CaC2 + CO
All these carbides react with water producing acetylene gas.
CaC2 + 2H2O → HC ≡ CH + Ca (OH)2
Reaction with Halogens
The alkaline earth metals react with halogens at elevated temperature to form the halides of the types MX2.
Action of Acids
The alkaline earth metals readily react with acids liberating hydrogen.
Reaction with Ammonia
Like alkali metal, the alkaline earth metals dissolve in liquid ammonia to give deep blue black solution from which ammoniates [ M (NH3)6 ]2+ can be recovered.
Solved Problem |
|
Question How does the basicity of oxides of group 2 increases down the group? Solution: The basicity increases down the group BeO < MgO < CaO < SrO < BaO amphoteric strongly - basic |
Formation of Peroxides
Since larger cations stabilize larger anions. Therefore, tendency to form peroxide increases as the size of the metal ion becomes larger. Thus BaO2 is formed by passing air over heated BaO at 773K.
 2BaO2
2BaO2  2SrO2
2SrO2 SrO2 is prepared in similar way but under high pressure and temperature. CaO2 is not formed this way but can be prepared as the hydrate by treating Ca (OH)2 with H2O2and then dehydrating the product.
Crude MgO2 has been made using H2O2 but peroxide of beryllium is not known.
All peroxide are white crystalline ionic solids containing the peroxide ion O2-2. Treatment of peroxide with acids liberates H2O2.
BaO2 + 2HCI → BaCI2 + H2O2
Reaction With Water (Formation of Hydroxides)
The electrode potential of Be (Be2+/Be = -1.97 V) is least negative amongst all the alkaline earth metals. This means that Be is much less electropositive than other alkaline earth metals and hence does not react with water or steam even at red heat.
The electrode potential of Mg (Mg+2/Mg = -2.37 V), although more negative than that of Be yet is still less negative than those of alkali metals and hence it does not react with cold water but reacts with boiling water or steam.
or, Mg + 2H2O → Mg (OH)2 + H2
Ca, Sr and Ba have more negative electrode potentials similar to those of the corresponding group I alkali metals and hence react with even with cold water, liberating H2 and forming the corresponding metal hydroxides.
Reactivity of alkaline earth metals increases as we move down the group. However, the reaction of alkaline earth metals is less vigorous as compared to alkali metals.
Reaction With Air (Nitrogen and Oxygen)
Formation of oxides and nitrides
Be metal is relatively unreactive in the massive form and hence does not react below 873K. However, powdered Be is more reactive and burns brilliantly on ignition to give a mixture of BeO & Be3N2.
 MH2 2BeO
MH2 2BeO  MH2 Be3N2
MH2 Be3N2magnesium is more electropositive than Be and hence burns with dazzling brilliance in air to form a mixture of MgO and magnesium nitride.
 MH2 MgO + Ng3N2
MH2 MgO + Ng3N2 Ca, Sr and Ba being even more electropositive react with air readily to form a mixture of their respective oxides and nitrides.
The reactivity towards oxygen increases as we go down the group. Thus Ca, Ba and Sr are stored in paraffin but Be and Mg are not because they form protective oxide layer on their surface.
Formation of Nitrides
All the alkaline metals burn in dinitrogen to form ionic nitrides of the formula, M3N2. This is in contrast to alkali metals where only Li forms Li3N.
 MH2 M3N2
MH2 M3N2Be3N2 being covalent is volatile while the nitrides of all other elements are crystalline solids.
All these nitrides decompose on heating and react with water liberating NH3.
Be3N2 M + H2  MH2 3Be + N2
MH2 3Be + N2
Ba3N2 + 6H2O M + H2  MH2 3Ba (OH)2 + 2NH3
MH2 3Ba (OH)2 + 2NH3
Ca3N2 + 6H2O M + H2  MH2 3Ca (OH)2 + 2NH3
MH2 3Ca (OH)2 + 2NH3

Question 1: Which of the following alternatives represents the correct order of soluble of sulphates of alkali earth metals in water ?
a. BeSO4 > MgSO4 > CaSO4 > SrSO4 > BaSO4
b. MgSO4 > BeSO4 > CaSO4 > SrSO4 > BaSO4
c. SrSO4 > MgSO4 > CaSO4 > BeSO4 > BaSO4
d. CaSO4 > SrSO4 >BeSO4 > MgSO4 > BaSO4
Question 2: Which of the following earth metals do not impart colour to the flame?
a. Sr
b. Ca
c. Mg
d. Ba
Question 3: Which of the following alternatives do not represent the correct property of alkali earth metals?
a. Hydroxides of alkali earth metals are weak bases, less soluble and decompose on heating.
b. Carbonates of alkali earth metals are insoluble in water and decompose on heating.
c. Nitrates of alkali earth metals decompose on heating evolving a mixture of NO2 and oxygen.
d. Alkali earth metals are stronger reducing agents than alkali metals as ionization potential values are low and oxidation potential values are high.
Question 4: Which of the following alternatives represents on of the products of the reaction
CaC2 + 2H2O →
a. HC ≡ CH
b. CaO
c. CaC

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
c |
d |
a |
Related Resources
-
Look here for past year papers of IIT JEE
-
Click here to refer syllabus of chemistry for IIT JEE
To read more, Buy study materials of S- Block elements comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
Contact askiitians experts to get answers to your queries by filling up the form given below:
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More