Allotropes of Carbon
Table of Content |
|
|
Allotropes and Allotropy
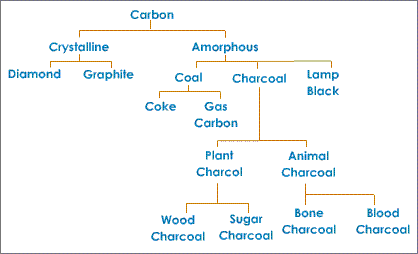
The presence of one element in various structures, having distinctive physical properties, however comparable chemical properties are known as Allotropy. Diverse types of an element are called "Allotropes" or Allotropic Structures. Carbon demonstrates allotropy. The different allotropic types of carbon can be extensively characterized into two classes, namely: Crystalline form and Amorphous form.
Fig. 2: Different allotropes of carbon: (a) graphite, (b) graphene, (c) carbon nanotube, (d) C60, (e) C70, (f) C540, (g) amorphous carbon, (h) lonsdaleite, and (i) diamond.
Crystalline Form
There are two types of crystalline forms: Diamond and Graphite.
 |
 |
Fig. 3: Diamond |
Fig. 4: Graphite |
Amorphous Form
Amorphous forms of carbon are: Coke, Coal, Charcoal (or wood charcoal), Lamp black, Animal charcoal (or bone black), Carbon black, Petroleum coke, Gas carbon are amorphous forms of carbon.
Diamond
Fig. 5: Internal Structure of Diamond
Diamonds are mainly found in the Union of South Africa, Brazil, the Belgian Congo, Brazil, India, British Guiana, and so forth.
Diamond was found without precedent for India. The well known 'Kohinoor precious stone' (186 - carat) and the 'Regent or Pitt' (studded in Napoleon's state sword, 136.2 carat) were found close Kishna waterway in South India.
The 'Cullinan diamond', the biggest ever discovered weighed 3025.75 carat (around 600 g) was mined in South Africa in 1905.
Diamonds exist as transparent octahedral precious stones mostly having bended surfaces and don't sparkle much in their characteristic natural frame. To give them their standard splendid sparkle they are cut at an appropriate point in order to offer ascent to huge total internal reflections.
Moissan (1893) developed the first artificial diamond by warming pure sugar charcoal and iron in a graphite pot to a temperature of around 3000°C in an electric circular segment furnace.
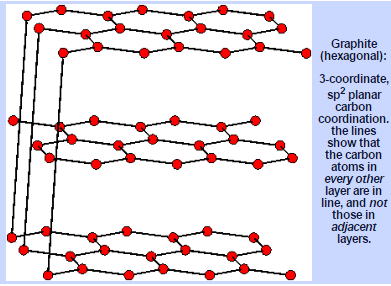
Graphite
Graphite is discovered broadly dispersed in nature, viz., in Sri Lanka, Siberia, Canada, USA.
Extensive amounts of graphite are additionally made from coke or anthracite in electric furnaces.
Diamonds and graphite are two crystalline allotropes of carbon. Diamond and graphite both are covalent gems. Be that as it may, they vary significantly in their properties.
Fig. 6: Internal Structure of Graphite
Diamond |
Graphite |
|
It occurs naturally in fee state |
It occurs naturally and is manufactured artificially |
|
It is the hardest natural substance known |
It is soft and greasy to touch |
|
It has high relative density (about 3.5) |
Its relative density is 2.3 |
|
It is transparent and has high refractive index (2.45) |
It is black in colour and opaque |
|
It is bad-conductor of heat and electricity |
Graphite is a good conductor of heat and electricity |
|
It burns in air at 900°C to give CO2 |
|
|
It occurs as octahedral crystals |
It occurs as hexagonal crystals |
|
It is insoluble in all solvents |
It is insoluble in all ordinary solvents |
These distinctions in the properties of diamond and graphite are because of the distinctions in their structures.
Structure of Diamond
In diamond, the carbon molecules are organized tetrahedrally (sp3 hybridisation of C): every C ion is connected to its neighbors by four single covalent bonds. This prompts to a three-dimensional system of covalent bonds.
Fig: 7: Structure of Diamond
Fig. 8: Structural Aspects of Diamond
It is because of this, that diamond is hard, and has high melting and boiling points. In diamond, every carbon particle is bonded to the next through customary covalent bonds. The electrons in this way are held firmly between the cores, and there are no portable electrons for the conduction of electricity i.e. all the valence electrons of carbon are spent in framing the covalent bonds. Subsequently diamond is not a conductor of electricity. Diamond is likewise denser than graphite (thickness: Diamond = 3.52 g cm-3 Graphite =2.25 g cm-3) as the Diamond structure is a firmly pressed structure, while the layer-to-layer extensive separation makes graphite to have an open structure.
Structure of Graphite
In graphite, the carbon molecules are displayed in level parallel layers as consistent hexagons. Every layer is bonded to contiguous layers by weak Vander Waals strengths. This permits every layer to slide over the other effectively. Because of this sort of structure graphite is delicate and slippery, and can go about as a lubricant. Graphite is additionally a good conductor of electricity. In graphite, carbon iotas in every layer are bonded to three other carbon molecules by unique covalent bonds. This gives some double bond character to the C-C bonds. This gives it the nearness of delocalized p-electron framework. These versatile electrons lead to the electrical conductivity of graphite.
Fig. 9: Structural Aspects of Graphite
Uses of Diamond
-
The splendid sparkle of diamond makes it an important valuable stone in adornments.
-
Making high-precision cutting devices for use in the therapeutic field.
-
Because of its hardness, it is utilized as a part of assembling apparatuses / cutting drills for cutting rocks and glass.
-
In making colors for drawing dainty wires of harder metals. Tungsten wires of thickness 1/sixth that of human hair can be drawn by utilizing diamond dyes.
Uses of Graphite
-
As a refractory material of making cauldrons and terminals for high-temperature work.
-
In electrotyping and in the fabrication of gramophone records: Graphite is utilized for making the non- conductor (for the most part wax) surface, so that electroplating becomes possible.
-
For producing lead pencils and stove paints.
Amorphous Forms of Carbon
A few amorphous forms of carbon are discussed below:
Coal
Coal is shaped in nature by the carbonization of wood. Change of wood to coal affected by high pressure, high temperature and without air is named carbonization.
Among coal assortments, anthracite is the purest frame. It consists of about 94-95% of carbon. The basic assortment is bituminous coal; it is dark, hard and ignites with smoky fire.
Uses
Coal is essentially utilized,
-
As a mechanical fuel in steel, power generating plants and so on. It is additionally a domestic fuel to a restricted degree.
-
For the manufacture of water gas and producer gas, which are utilized as fuel gases.
-
For fabricating coke, coal tar and coal gas.
-
Anthracite coal is utilized for producing graphite.
-
For the fabrication of manufactured petrol by catalytic hydrogenation of coal.
Wood Charcoal
At the point when wood is warmed emphatically in an exceptionally constrained supply of air, wood charcoal is acquired. This is termed as the destructive distillation of wood. The volatile items are permitted to get away.
Charcoal is a dark, permeable and weak solid. It acts as a good adsorbent. Charcoal powder adsorbs shading matter from solutions and harmful gasses from the air. Charcoal is likewise a good reducing agent.
-
As a fuel.
-
As an antiperspirant and in gas masks to filter contamination.
-
As a staining operator for decolorizing oils, and so on.
-
In making gun powder.
Animal Charcoal
Animal charcoal (or Bone charcoal) is acquired by destructive distillation of bones. It consists of about 10-12% of amorphous carbon.
Sugar Charcoal
It is acquired by warming sugar without air. Sugar charcoal is the purest type of amorphous carbon.
Sugar charcoal gets to be distinctly activated charcoal when it is powdered to molecule size of around 5 micron and warmed at around 1000 K in vacuum. Activated charcoal has an expanded adsorption limit.
Lamp Black
Lamp Black is fabricated when tar and vegetable oils (rich in carbon) are singed in a deficient supply of air and the subsequent residue is deposited on wet blankets in a room.
Lamp Black is a velvety black powder. It is utilized as a part of the preparation of Indian ink, carbon papers, printer's ink, varnishes, and black paint.
Carbon Black
At the point when natural gas is burnt in constrained supply of air, the subsequent sediment is kept on the underside of a spinning plate. This is carbon black and it is then scratched off and filled in sacks.
It varies from lamp black in being not all that greasy. Carbon black is added to the rubber blend utilized for making car tyres, and has substituted the utilization of lampblack for various purposes.
Gas Carbon and Petroleum Coke
Carbon scratched from the dividers of the retort utilized for the destructive refining of coal is called Gas Carbon. Amid refining of crude petroleum, petroleum coke is stored on the dividers of the refining tower.
Both, gas carbon and petroleum coke are utilized for making terminals in dry cells and are great conduits of power.
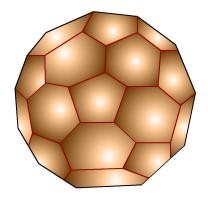
Fullerenes
Fullerenes are as of late discovered (1985) allotropes of carbon. They have been found to exist in the interstellar dust and in addition inland developments on earth. They are substantial cage- like round particles with formulae C32, C50, C60, C70, C76, C84 and so forth. The most ordinarily referred to fullerene is C60 which is named as 'buckminster fullerene' after the creator of the geodesic vault, American modeler Buckminster.
Structure of C60 Particle
C60 particle has gloriously symmetrical structure. It is an intertwined ring of aromatic framework containing 20 hexagons and 12 pentagons of sp2 hybridized C iotas. The structure twists around and closes to frame a soccer ball molded particle. C60 is along these lines, called buckyball too. The width of ball confine is around 70 pm. It is around 6-10 times as huge as H molecule may be. The cages of ball are very stable and don't separate till 1375 K. It is an exceedingly symmetrical structure in which all the carbon molecules possess indistinguishable position.
Fig. 10: Buckminster Fullerene (C60)
Fig. 11: Structure of C- 60
Appearance and Applications
C60 fullerene does not look the same as diamond and graphite. It is a yellow fine substance, which becomes pink on disintegration over solvents like toluene. It polymerizes on introduction to U.V. radiations.
Fullerenes are entrancing on the grounds that they indicate unusual attributes and applications like:
-
They are awesome lubricants on the grounds that the balls can move between the surfaces.
-
Alkali compounds of C60 (A3C60) are super conductor materials even at high temperatures of the order of 10-40 K.
Q1. Diamond is extremely unreactive at room temperature, but graphite reacts more readily, why?
Sol. In diamond, all the four valencies of every carbon particle are fulfilled by bonding to other carbon molecules through normal covalent bonds. The electrons are held firmly between the cores, and there are no versatile electrons. Along these lines diamond structure is a firmly stuffed structure and is to a great degree unreactive at room temperature.
In graphite, carbon molecules in every layer are bonded to three carbon iotas. This gives some double bond character to C-C bonds giving it the nearness of delocalized p-electron framework. The layer-to-layer vast separation makes graphite to have an open structure thus it reacts all the more promptly.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free