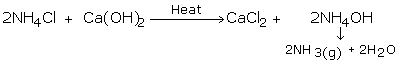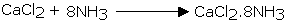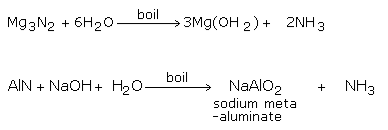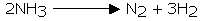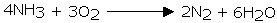Ammonia
Table of Content |
Occurrence
Ammonia (NH3) is an essential compound of nitrogen and hydrogen. It is created by the regular decay of vegetable and animal bodies. The demise and rot of animals and plants cause the nitrogen compounds exhibit in them to get deteriorated, producing ammonia. Ammonia likewise is present in the soil as ammonium salts.
Preparation of Ammonia
Ammonia can be manufactured by the following methods:
From Ammonium Chloride
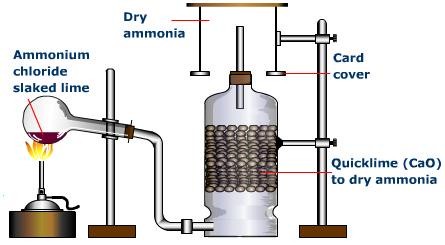
Ammonia gas is generally manufactured in the research center by slowly heating ammonium chloride (NH4Cl) and slaked lime [Ca(OH)2].
Fig. 1: Preparation of Ammonia
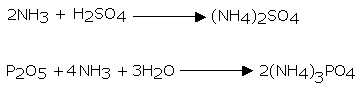
Ammonia gas is lighter than air, requiring its accumulation by the descending displacement of air. Since it is quite solvent in water it can't be gathered over it. Advancing ammonia gas through quicklime (CaO) dries it. Being an essential gas, ammonia can't be dried by advancing it through concentrated sulphuric acid or phosphorus pentoxide (P2O5), since it reacts with them to frame ammonium sulfate or ammonium phosphate separately.
Calcium chloride additionally can't be utilized for drying ammonia gas as it structures ammoniates withCaCl2
By the Hydrolysis of Metal Nitrides
Hydrolyzing metal nitrides like magnesium and aluminum nitrides, with water or alkalis, can likewise deliver Ammonia gas.
Manufacture of Ammonia
Haber's Process
The preparation of ammonia by Haber's procedure includes direct mixing of nitrogen and hydrogen.
This reaction is, (a) exothermic, (b) reversible, and (c) proceeds with a decrease in volume. As per the Le Chatelier's principle, the ideal conditions for the arrangement of ammonia are:
Low Temperature
The temperature ought to stay as low as would be prudent, (in spite of the fact that at strangely low temperatures, the rate of reaction turns out to be moderate). It has been found that the temperature, which upgrades the yield of ammonia for the reaction, is most extreme at about 500°C.
High Pressure
Since Haber's procedure continues with abatement in volume, it is supported by high pressure. In real practice, a pressure of 200 - 900 atmospheres is utilized.
Catalyst
An impetus is generally utilized to expand the speed of the reaction. Finely isolated iron containing molybdenum or alumina is utilized as an impetus. Molybdenum or alumina (Al2O3) goes about as a promoter and builds the productivity of the impetus. A blend of iron oxide and potassium aluminate has been found to work all the more adequately.
Fig. 2: Manufacturing Plant Employed in Haber's Process
Source of Raw Materials
The nitrogen and hydrogen gases utilized as the crude material in Haber's procedure are acquired in the following way.
-
Nitrogen is taken from the fluid air and hydrogen from water by electrolysis.
-
Hydrogen might be taken from water gas (blend of CO and H2) by Bosch process.
-
Water gas can be get by advancing steam over intensely hot coke.

By bubbling the mixture of water gas through water, CO2 is removed as shown above.
-
A mixture of carbon dioxide, hydrogen and nitrogen may be obtained by treating a mixture of water gas (CO + H2),producer gas (CO + N2), with steam in the presence of ferric oxide - chromium oxide catalyst at 450°C.

Carbon dioxide may be isolated by bubbling through water under pressure.
Plant
The following are the components and processes of the plant, which manufactures ammonia.
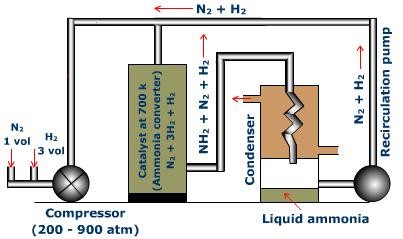
Compressor
A combination of hydrogen and nitrogen in the ratio 1: 3 (by volume) is compressed to 200-900 atmosphere pressure. The compressed gas is then sent to ammonia converter.
Fig.3: Ammonia Converter
Converter
Ammonia converter is produced using chrome-vanadium steel. It is normally 1.3 meters high and 1 meter in measurement. The converter is furnished with a warmth exchanger in the upper part and the impetus is packed in the focal segment of the converter. There is a process for warming the combination of gases. After the gas blend enters through the bay at the base, the gases course around the impetus kept up at 450-500°C and afterward goes through to the heat exchanger. The gases at the end enter the chamber of catalyst to give ammonia. Before entering the condensers the final product and additionally the unreacted gasses go through the channels of the warmth exchanger and exchange their warmth to the approaching mixture of gas containing nitrogen and hydrogen.
This cools and melts ammonia. The consolidated ammonia, called 'liquor ammonia' is filled into chambers under pressure.
Re-circulating Pump
A portion of the nitrogen and hydrogen gases escape condensation and are re-circled through the converter.
Structure of Ammonia
Ammonia is a covalent atom as is appeared by its dot structure. The ammonia particle is shaped because of the overlap of orbitals of three hydrogen and three sp3 hybrid orbitals of N in the structure as central atom. The fourth sp3 hybrid orbital is involved by a lone pair. This provides a trigonal pyramidal shape to ammonia particle. The H-N-H bond edge is 107.3°, which is somewhat not exactly the tetrahedral edge of 109°28. This is on the grounds that the bond pair- lone pair repulsions push the N-H bonds somewhat inwards. In solid and liquid states, ammonia is related through hydrogen bonds.
Fig. 4: Trigonal Pyramidal Shape of Ammonia
Fig. 5: Dot structure of Ammonia
Physical Properties of Ammonia
-
Ammonia is a gas that has no color.
-
It has a sharp pungent odor having a soapy taste. At the point when inhaled all of a sudden, it attacks the eyes bringing tears.
-
It is lighter than air and is in this way gathered by the descending displacement of air.
-
It is very soluble in water: One volume of water breaks up around 1300 volumes of ammonia gas. It is because of its high dissolvability in water due to hydrogen bonding that the gas cannot be collected over water.
-
It can be effortlessly melted at room temperature by applying a pressure of around 8-10 atmospheres.
-
Liquid ammonia bubbles at 239.6 K (- 33.5°C) under one-atmosphere pressure. It has a high value of the latent heat of vaporization (1370 J for each gram) and is subsequently utilized as a part of refrigeration plants of ice making machines.
-
Liquid ammonia solidifies at 195.3 K (- 77.8°C) to give a solid that is white crystalline in appearance.
Chemical Properties of Ammonia
Thermal Stability
Ammonia is exceptionally inert. In any case, it can be disintegrated into hydrogen and nitrogen by advancing over metallic impetuses that have been heated or when electric discharge is passed through it.
Combustibility
Ammonia is flammable in air. Nonetheless, it will blaze in the presence of oxygen
Nitric oxide is acquired when a blend of ammonia and air is advanced over platinum - rhodium impetusat 800°C
Basic Character
Ammonia particle has a solid propensity to give its lone pair of electrons of nitrogen to different atoms. Consequently, it acts like a strong Lewis base. In aqueous arrangements, NH3 ionizes as per the reaction.
The equilibrium constant at 298 K for this reaction is 1.8 x 10-5. Along these lines, ammonia ionizes to a little degree in aqueous solution. The fluid arrangement of ammonia goes about as a weak base because of the nearness of OH-ions. In this way, ammonia turns red litmus blue and reacts with acids to frame salts.
For Example,
With Metal Oxides
Ammonia when advanced over-heated metal oxides gets oxidized to nitrogen.
With Halogens
Ammonia reacts easily with halogens and the nature of products is determined by the type of halogen and reaction conditions.
Chlorine
With a restricted amount of chlorine, Nitrogen and ammonium chloride are formed. Similarly, nitrogen trichloride is formed in the presence of excess of chlorine.
Bromine
Nitrogen and ammonium bromide are formed
Iodine
Nitrogen tri-iodide, a dark colored precipitate is obtained when rubbed with solid iodine
After drying, an explosion of iodine vapors is produced if NH3.NI3 is hit with a hammer or struck against a hard surface.
With Carbon Dioxide (formation of urea)
Urea is produced when Ammonia is heated under pressure with CO2.
With Alkali Metals
Upon advancing ammonia over heated potassium or sodium, amides are formed liberating hydrogen.
Alkali metals give a blue solution when dissolved in liquid ammonia which when left undisturbed liberated hydrogen. The blue color of these solutions is a result of the presence of solvated electrons (e- (NH3)n). For Example, with sodium
Action with Heavy Metal Ions
Ammonia forms insoluble metal hydroxides that form precipitates with the metal ions of Al, Fe, Cr, and Zn.
Formation of Complex Compounds
Ammonia upon reaction with soluble salts of copper, silver etc.forms complex compounds. On reaction with copper sulphate solution, it gives a deep blue colored complex compound, tetramminecopper(II) sulphate.
Uses of Ammonia
-
In the production of urea and rayon
-
In the production of composts, for example, ammonium nitrate, urea diammonium phosphate, ammonium sulfate and so on.
-
As a refrigerant, in ice plants.
-
In the furniture industry, as a purging operator for furniture and glass surfaces.
-
In the production of nitric acid by Ostwald's procedure.
-
In the production of sodium carbonate by Solvay's procedure.
Tests of Ammonia
The under mentioned tests of any specimen affirm the presence of ammonia:
-
The ammoniacal odor of ammonia is effectively perceivable having a trademark pungent smell.
-
Ammonia turns wet red litmus blue and moist turmeric paper brown in color.
-
A glass bar dunked in concentrated HCl when conveyed near ammonia, causes thick white exhaust.
-
The ammoniacal smell of ammonia is easily detectable having a characteristic pungent smell.
-
Ammonia turns moist red litmus blue and moist turmeric paper brown.
-
When added to a solution of copper sulphate, ammonia turns the solution deep blue.
NH3 + CuSO4 + nH2O→ [Cu(NH3)4(H2O)n]SO4
-
When added to Nessler's reagent (basic arrangement of K2[HgI4] ammonia gives precipitate brown in color.
NH4+ + 2[HgI4]2− + 4OH− → HgO·Hg(NH2)I ↓ + 7I− + 3H2O
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More