Dioxygen
Table of Content |
General Discussion
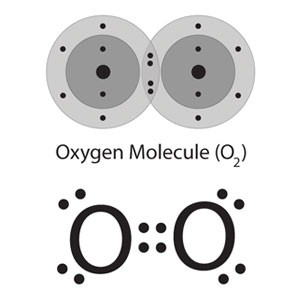
Similar to Group 14 and 15, the lightest member of group 16 has the best inclination to shape numerous bonds. In this way, Elemental Oxygen is found in nature as a diatomic gas that contains a net twofold bond: O=O. Dioxygen means the normal allotrope of oxygen having two atoms of oxygen in the molecule.
Fig. 1: Lewis Structure of Dioxygen
Fig. 2: Ball and Stick Model of Dioxygen
Likewise with nitrogen, electrostatic repulsion between lone pair of electrons on adjoining atoms keeps oxygen from framing stable catenated compounds. Truth be told, aside from oxygen, compounds that contain O–O bonds are conceivably hazardous. Ozone, superoxides, and peroxides are all possibly very harmful when present in their pure forms. Ozone (O3), a standout amongst the most intense oxidants known, is utilized to cleanse drinking water since it doesn't deliver the characteristic taste connected with chlorinated water. Hydrogen peroxide (H2O2) is so thermodynamically unsteady that it tends to experience unstable decay when in impure form.
Equation
2H2O2 (l) → 2H2O(l) + O2 (g) ΔG° = −119 kJ/mol
Regardless of the quality of the O = O bond (DO2 = 494 kJ/mol), O2 is amazingly reactive, that reacts straightforwardly with almost all different elements aside from the noble gasses. A few properties of O2 and related species, for example, the peroxide and superoxide particles, are appeared in the accompanying table:
Species |
Bond Order |
Number of unpaired e- |
O- O distance (pm) |
| O2 | 2 | 2 | 121 |
| O2+ | 2.5 | 1 | 112 |
| O2- | 1.5 | 1 | 133 |
| O22- | 1 | 0 | 149 |
Preparation of Dioxygen
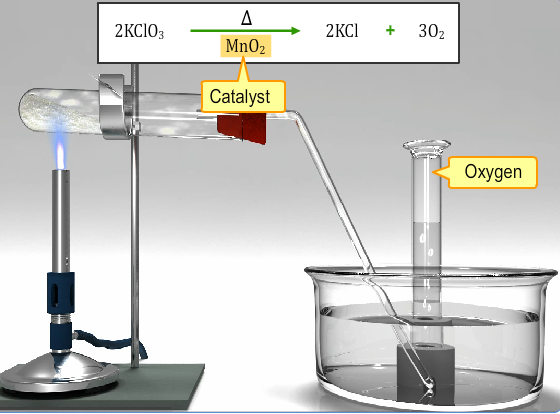
The most advantageous technique for making dioxygen in the laboratory includes the catalytic decay of potassium chlorate in solid form where manganese dioxide works as a catalyst.
Δ
2KClO3 → 2KCl + 3O2
MnO2
Fig. 3: Catalytic Decomposition of Solid Potassium Chlorate
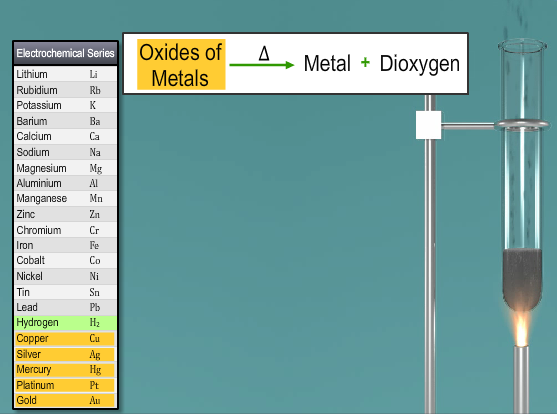
Another laboratory technique includes the thermal disintegration of metal oxides from the lower part of the electrochemical arrangement. Illustration: The thermal decomposition of mercuric oxide or silver oxide gives dioxygen.
2HgO → 2Hg + O2
Mercuric oxide Δ Mercury Dioxygen
2Ag2O → 4Ag + O2
Silver oxide Δ Silver Dioxygen
Fig. 4: Thermal Decomposition of Metallic Oxides
Dioxygen can also be prepared in the laboratory by thermal treatment of the higher oxides of some metals like lead, manganese and barium.
2PbO2 → 2PbO + O2
Lead (IV) oxide Δ Lead (II) oxide Dioxygen
2MnO2 + 2H2SO4 → 2MnSO4 + 2H2O + O2
Manganese Sulphuric Δ manganese Water Dioxygen
(IV) oxide acid (II) sulphate
2BaO2 → 2BaO + O2
Barium peroxide Δ Barium oxide Dioxygen
Salts that are rich in oxygen, such as permanganates and nitrates,when decomposed thermally also yields dioxygen.
2KNO3 → 2KNO2 + O2
Potassium Δ Potassium Dioxygen
nitrate nitrate
2KMnO4 → K2MnO4 + MnO2 + O2
Potassium Δ Potassium manganese Dioxygen
permanganate manganate (IV) oxide
2NaNO3 → 2NaNO2 + O2
Sodium Δ Sodium Dioxygen
nitrate nitrate
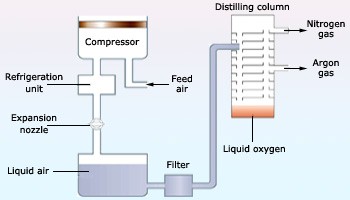
Industrially, preparation of dioxygen includes electrolysis of air or fractional distillation of liquid air.
Fig. 5: Fractional Distillation of Liquid Air
Physical Properties of Dioxygen
-
Dioxygen is a tasteless, colorless and scentless gas.
-
It is marginally heavier than air.
-
It is marginally soluble in water. This little amount of dioxygen dissolved is quite adequate to support the aquatic and marine life.
Under pressure, it can be condensed to a light blue fluid by compacting the gas at 90K. It can likewise be solidified into a bluish white solid at 55K.
Fig. 6: Dioxygen 2-d Dimensions
Chemical Properties of Dioxygen
Dioxygen is an exceptionally reactive element and reacts straightforwardly with almost all metals and non-metals. It doesn't react straightforwardly with a few metals like gold and platinum and some noble gases like helium, argon, and neon.
Reaction of Dioxygen With Metals
Most metals blaze in dioxygen and frame oxides that are for the most part basic in nature.
Metal Dioxygen Metal-oxide
2M + O2 → 2MO
Δ
4M + O2 → 2M2O
Δ
4M + 3O2 → 2M2O3
Δ
A large portion of non-metals burn within thepresence of dioxygen structures acidic oxides. Example: Sulfur blazes within the presence of oxygen gives sulfur dioxide.
Δ
S + O2 → SO2
Reactions of Dioxygen With Some Compounds
Sulfur dioxide experiences catalytic oxidation within the presence of vanadium pentoxide to frame sulfur trioxide. This is a critical stride in the production of sulphuric acid by the contact procedure.
V2O5
2SO2 + O2 → 2SO3
Dioxygen reacts with many organic compounds such as carbohydrates and hydrocarbons, at hoisted temperatures or on start, resulting in the formation of carbon dioxide and water.
High Temperature
CH4 + 2O2 → CO2 + 2H2O
Methane Dioxygen Carbon Water
dioxide
High Temperature
C6H12O6 + 6O2 → 6CO2 + 6H2O
Glucose Dioxygen Carbon Water
dioxide
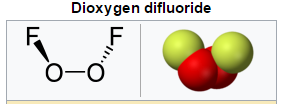
Dioxygen Difluoride
Dioxygen Difluoride with the formula O2F2 is a compound of fluorine and oxygen. It can exist as an orange-colored solid which liquefies into a red fluid at −163 °C (110 K). It is to a great degree solid oxidant and breaks down into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% every day: its lifetime at room temperature is in this manner amazingly short. Dioxygen difluoride reacts energetically with about each concoction it experiences – even common ice – prompting to its onomatopoeic label "FOOF."
Fig. 7: Structure of Dioxygen Difluoride
Uses of Dioxygen
-
The fundamental significance of dioxygen lies in its support to key procedures. For Example: respiration and combustion.
-
Dioxygen blended with carbon dioxide or helium is utilized for artificial respiration.
-
It is utilized as a part in manufacturing many metals.
-
It is utilized as a part of oxy-acetylene welding and metal cutting.
-
It is utilized to oxidize ammonia in the nitric acid preparation.
-
Oxygen barrels are broadly utilized as a part of healing facilities, high-height flying and in mountaineering.
-
Liquid oxygen is an essential constituent of the fuel utilized as a part of rockets.
Frequently Asked Questions (FAQs)
Q1. What is Dioxygen?
Sol. The basic allotrope of elemental oxygen on Earth, O2, is for the most part known as oxygen, however might be called dioxygen or sub-atomic oxygen to recognize it from the element itself. Elemental oxygen is most regularly experienced in this frame, as around 21% (by volume) of Earth's air.
Q2. How do they make oxygen?
Sol. In nature, free oxygen is delivered by the light-determined part of water amid oxygenic photosynthesis. As indicated by a few evaluations, green growth and cyanobacteria in marine situations give around 70% of the free oxygen delivered on Earth and the rest is created by earthbound plants.
Q3. What is atomic oxygen gas?
Sol. Atomic oxygen, denoted O(3P), O(3P) or O((3)P), is extremely reactive, as the single particles of oxygen tend to rapidly bond with adjacent particles; on Earth's surface it doesn't exist normally for long, however in space, the nearness of a lot of bright radiation brings about a low Earth orbit environment in which 96% of the oxygen happens in nuclear form.
Q4. How was oxygen discovered as an element?
Sol. Oxygen was found in 1774 by Joseph Priestley in England and two years prior, yet unpublished, via Carl W. Scheele in Sweden. Scheele heated a few compounds including potassium nitrate, manganese oxide, and mercury oxide and discovered they discharged a gas which improved burning. Joseph Priestley and Carl Wilhelm Scheele both freely found oxygen, however Priestly is generally given acknowledgment for the revelation
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free