General Characteristic of the Compounds of the Alkali Metals
Table of Content |
|
|
Oxides and Hydroxides
 All the alkali metals, their oxides, peroxides and superoxides readily dissolve in water to produce corresponding hydroxides which are strong alkalies eg
All the alkali metals, their oxides, peroxides and superoxides readily dissolve in water to produce corresponding hydroxides which are strong alkalies eg
-
2Na + 2H2O → 2NaOH + H2
-
Na2O + 2H2O → 2NaOH
-
Na2O2 + 2H2O → 2NaOH + H2O2
-
2KO2 + 2H2O → 2KOH + H2O2 + O2
Thus peroxides and superoxides also act as oxidizing agents since they react with H2O forming H2O2 and O2 respectively.
The hydroxides of all the alkali metals are white crystalline solids. They are strongest of all base and readily dissolve in water with the evolution of much heat.
Watch this Video for more reference
Basic Strength
-
The basic strength of these hydroxides increases as we move down the group Li to Cs.
-
The hydroxides of alkali metals behave as strong bases due to their low ionization energies which decrease down the group.
-
The decrease in ionization energies leads to weakening of the bond between metal and hydroxide ion and M – O bond in M – O – H can easily break giving M+ and OH- .
-
This results in the increased concentration of hydroxyl ions in the solution i.e increased basic characters.
Solubility and Stability
All these hydroxides are highly soluble in water and thermally stable except lithium hydroxide.
2LiOH +Δ → Li2O + H2O
Formation of Salts with Acids
Alkali metals hydroxides being strongly basic react with all acids forming salts.
-
NaOH + HCI → NaCI + H2O
-
2NaOH + H2SO4 → Na2SO4 + 2H2O
Halides of Alkali Metals
The alkali metals combine directly with halogens under appropriate conditions forming halides of general formula MX. These halides can also be prepared by the action of aqueous halogen acids (HX) on metals  oxides, hydroxides or carbonate.
oxides, hydroxides or carbonate.
-
M2O + 2HX → 2MX + H2O
-
MOH + HX → MX + H2O
-
M2CO3 + 2HX → 2MX + CO2 + H2O (M = Li, Na, K, Rb or Cs)
-
(X = F, Cl, Br or I)
All these halides are colourless, high melting crystalline solids having high negative enthalpies of formation.
|
Standard enthalpies of formation in (kJ/mol-1) |
||||
|
Element |
MF |
MCl |
MBr |
MI |
|
Li |
-612 |
-398 |
-350 |
-271 |
|
Na |
-569 |
-400 |
-360 |
-288 |
|
K |
-563 |
-428 |
-392 |
-328 |
|
Rb |
-549 |
-423 |
-389 |
-329 |
|
Cs |
-531 |
-424 |
-395 |
-337 |
The value decreases in the order:
Thus fluorides are the most stable while iodides are the least stable.
The trends in melting points, boiling points and solubility of alkali metals halides can be understood in terms of polarization effects, lattice energy and hydration of ions.
Polarization Effects
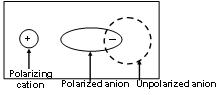 Comparison of ionic and covalent character of alkali metal halides. When a cation approaches an anion, the electron cloud of the anion is attracted towards the cation and hence gets distorted. This effect is called polarization. The power of the cation to polarize the anion is called its polarizing power and the tendency of the anion to get polarized is called its polarizability. The greater the polarization produced more is the concentration of the electrons between the two atoms thereby decreasing the ionic character or increasing the covalent character. The covalent character of any compound in general depends upon the following factors.
Comparison of ionic and covalent character of alkali metal halides. When a cation approaches an anion, the electron cloud of the anion is attracted towards the cation and hence gets distorted. This effect is called polarization. The power of the cation to polarize the anion is called its polarizing power and the tendency of the anion to get polarized is called its polarizability. The greater the polarization produced more is the concentration of the electrons between the two atoms thereby decreasing the ionic character or increasing the covalent character. The covalent character of any compound in general depends upon the following factors.
-
Size of the Cations
Smaller the cation greater is its polarizing power and hence larger is the covalent character. The covalent character decreases as size of cation increases.
LiCl > NaCl > KCl > RbCl > CsCl
- Size of the Anion
Larger the anion, greater is its polarizability. This explains the covalent character of lithium halides is in order
- Charge of the Ion
Greater the charge on the cation greater is its polarizing power and hence larger is the covalent character. The covalent character of some halides increase in the order
Na+Cl- < Mg+2Cl2 < Al+3 Cl3
Similarly greater the charge on the anion, more easily it gets polarized thereby imparting more covalent character to the compound formed eg covalent character increase in the order
NaCI < Na2SO4 < Na3PO4
Thus the covalent character decreases as the charge of the anion decrease.
-
Electronic configuration of the Cation
If two cations have the same charge and size, the one with pseudo noble gas configuration i.e. having 18 electrons in the outermost shell has greater polarizing power than a cation with noble gas configuration i.e having 8 electrons. For example CuCl is more covalent than NaCl.
Lattice Energies
Lattice energy is defined as the amount of energy required to separate one mole of solid ionic compound into its gaseous ions. Evidently greater the lattice energy, higher is the melting point of the alkali metals halide and lower is its solubility in water
|
Compound |
Lattice energy |
Hydration* energy |
Solubility |
Melting point |
|
LiCl |
-845 |
-876 |
63.7 |
887 |
|
NaCl |
-770 |
-776 |
35.7 |
1084 |
|
KCl |
-703 |
-700 |
34.7 |
1039 |
|
RbCl |
-674 |
-680 |
77.0 |
988 |
|
CsCl |
-644 |
-646 |
162 |
925 |
|
NaF |
-893 |
-919 |
4.22 |
1261 |
|
NaCl |
-770 |
-776 |
35.7 |
1028 |
|
NaBr |
-730 |
-745 |
116 |
1084 |
|
NaI |
-685 |
184 |
944 |
|
|
LiF |
-1005 |
-1019 |
0.27 |
1115 |
|
CsI |
-582 |
-670 |
44.0 |
1115 |
Hydration Energy
 It is the amount of energy released when one mole of gaseous ions combine with water to form hydrated ions.
It is the amount of energy released when one mole of gaseous ions combine with water to form hydrated ions.
-
M+ (g) + aq → M+ (aq) + hydration energy
-
X- (g) + aq → X- (aq) + hydration energy
-
Higher the hydration energy of the ions greater is the solubility of the compound in water.
-
Further the extent of hydration depends upon the size of the ions. Smaller the size of the ion, more highly it is hydrated and hence greater is its hydrated ionic radius and less is its ionic mobility (Conductance).
-
From above arguments, the melting point and solubility in water or organic solvent of alkali metal halides can be explained
-
A delicate balance between lattice enthalpy and hydration enthalpy determines the ultimate solubility of a compound in water. For eg. Low solubility of LiF (0.27 g/100 g H2O ) is due to its high lattice energy ( - 1005 KJmol-1) whereas the low solubility of CsI (44g/100g H2O ) is due to smaller hydration energy of the two ions (-670 KJ/mol) . The solubility of the most of alkali metal halides except those of fluorides decreases on descending the group since the decrease in hydration energy is more than the corresponding decrease in the lattice energy.
-
Due to small size and high electronegativity, lithium halides except LiF are predominatantly covalent and hence are soluble in covalent solvents such as alcohol, acetone, ethyl acetate, LiCl is also soluble in pyridine. In contrast NaCl being ionic is insoluble in organic solvents.
-
Due to high hydration energy of Li+ ion, Lithium halides are soluble in water except LiF which is sparingly soluble due to its high lattice energy.
-
For the same alkali metal the melting point decreases in the order fluoride > chloride > bromide > iodide because for the same alkali metal ion, the lattice energies decreases as the size of the halide ion increases.
-
For the same halide ion, the melting point of lithium halides are lower than those of the corresponding sodium halides and thereafter they decrease as we move down the group from Na to Cs.
-
The low melting point of LiCl (887 K) as compared to NaCl is probably because LiCl is covalent in nature and NaCl is ionic.
|
Solved Example |
|
Why are alkali metal halides soluble in water? Solution: Alkali metal halides are soluble in water due to their high ionic character and low lattice energy. |
Salts of Oxoacids
Since the alkali metals are highly electropositive, therefore their hydroxides are very strong bases and hence they form salts with all oxoacids ( H2CO3, H3PO4, H2SO4, HNO3, HNO2 etc) . They are generally soluble in water and stable towards heat. The carbonates (M2CO3) of alkali metals are remarkably stable upto 1273 K, above which they first melt and then eventually decompose to form oxides. Li2CO3 , however is considerably less stable and decomposes readily.
Li2CO3 + Δ→ Li2O + CO2
This is presumably due to large size difference between Li+ and CO2-3 which makes the crystal lattice unstable.
Being strongly basic, alkali metals also form solid bicarbonates. No other metals forms solid bicarbonates though NH4HCO3 bicarbonate though it does exist in solution. All thealso exists as a solid. Lithium, however does not form solid
2MHCO3 → M2CO3 + CO2 + H2O
|
Solved Example |
|
Question: Complete and balance the following: NaNO3 +Heat → 4LiNO3 → 2Li2O + 4NO2 + O2 2NaNO3 → 2NaNO2 + O2 |
Some other Compounds of Alkali Metals
Sodium Bicarbonate
A concentrated solution of sodium carbonate absorbs CO2 to give sparingly soluble sodium bicarbonate.
Properties of sodium bicarbonates
-
It is sparingly soluble in water
-
When heated between 250°C and 300°C, it is converted into pure anhydrous sodium carbonate which can be used for standardising acids. 2NaHCO3 → Na2CO3 + H2O + CO2
Potassium Bicarbonate
It is made by absorbing CO2 in moist potassium carbonate and then drying the product in a porous plate.
Properties of potassium bicarbonates
KHCO3 resembles NaHCO3, but is much more soluble in water. The solution is strongly alkaline owing to hydrolysis.
Sodium Chloride (NaCl)
It is also called common salt occurs abundantly in nature as rock salt or halite. The most abundant source is sea-water where sodium chloride occurs to the extent of 2.6 – 2.9 percent. The sea water is exposed to the sun and air in large shallow pits. The gradual evaporation of water leading to the crystallization of the salt. The purification is done by dissolving the salt in minimum volume of water and filtering, if necessary, to remove insoluble impurities. The solution is then saturated with a current of dry hydrogen chloride whereby crystals of pure sodium chloride separate out.
Properties of sodium chloride
-
NaCl is a colourless crystalline salt, almost insoluble in alcohol and highly soluble in water.
-
It gives rise to HCl when heated with conc. H2SO4 and Cl2, with MnO2 plus H2SO4.
NaCl + H2SO4 → NaHSO4 + HCl ↑
NaHSO4 + NaCl →Na2SO4 + HCl ↑
2NaCl + MnO2 + 2H2SO4 → MnSO4 + Na2SO4+ 2H2O + Cl2 ↑
Potassium Chloride
KCl is prepared from fused carnallite – nearly pure KCl separates from the melt, leaving fused MgCl2 behind.
KCl, MgCl2×6H2O → KCl + MgCl2×6H2O
Properties of potassium chloride
It is colourless cubic crystal like solid soluble in water. Its solubility increases almost linearly with temperature.
Sodium Sulphate, Na2SO4
The anhydrous salt known as salt cake, is prepared on an industrial scale by heating strongly sodium chloride with conc. sulphuric acid.
NaCl + H2SO4 → NaHSO4+ HCl ↑
NaCl + NaHSO4 → Na2SO4 + HCl ↑
Glauber’s salt or hydrated sodium sulphate, Na2SO4×10H2O is prepared from salt cake by crystallisation from water below 32°C This temperature represents the transition temperature for Na2SO4 and Na2SO4.10H2O.
It is colourless salt, crystallising in large monoclinic prisms. It is exceedingly soluble in water.
Potassium Sulphate, K2SO4
It is obtained by strongly heating potassium chloride with conc. H2SO4
KCl + KHSO4 → K2SO4+ HCl
It is colourless crystalline salt, m.p. 1070°C. It is less soluble in water than sodium sulphate and has no hydrate like the later.

Question 1: Na2O + H2O →
a. NaOH
b. Na2O2
c. NaO
d. Na2O
Question 2: Which of the following alkali metals do not for a stable oxide?
a. Li
b. Na
c. K
d. Rb
Question 3: Which of the following halides have highest lattice enthalpy?
a. NaCl
b. LiCl
c. KCl
d. RbCl
Question 4: For the same alkali metal the melting point of halides decreases in the order
a. chloride > bromide > iodide >fluoride
b. chloride > fluoride > bromide > iodide
c. fluoride > chloride > bromide > iodide
d. bromide > fluoride > chloride > iodide

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
a |
a |
b |
c |
Related Resources
-
Look here for past year papers of IIT JEE
-
Click here to refer syllabus of chemistry for IIT JEE
-
You can also refer to alkali metals
To read more, Buy study materials of S- Block elements comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More