Group 16 Elements
Table of Content |
|
|
Group 16 in the p block is the first group which has no stable metallic elements.
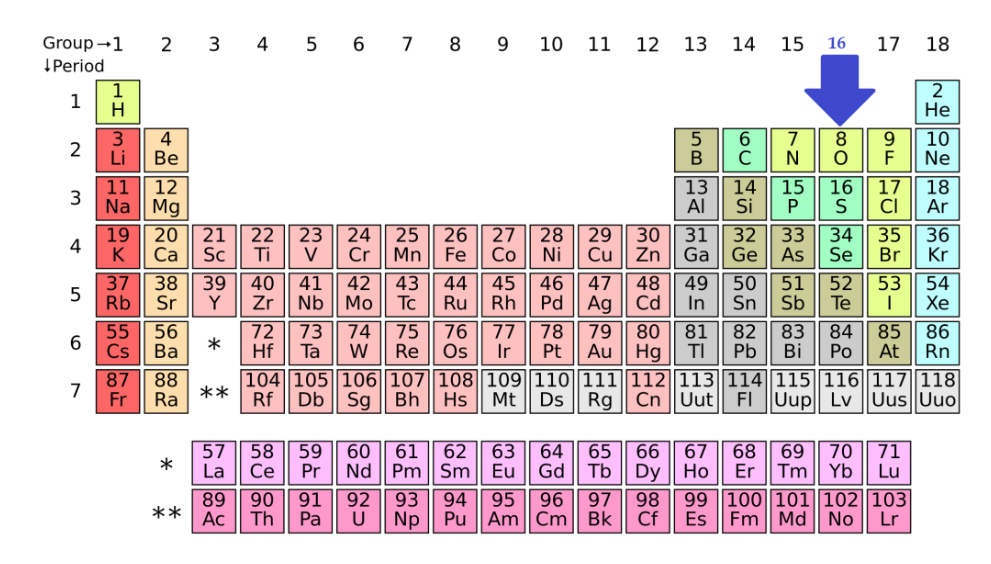
Fig. 1: Position of Group 16 Elements in Periodic table
Occurrence and General Characteristics
The elements oxygen, sulfur, selenium, tellurium and polonium comprises of the 16th vertical column or VIA group elements in the currently used long type of periodic table.
Fig. 2: Elements of Group 16
The initial four elements of the group are together termed as chalcogens or ore-forming elements, on the grounds that an extensive number of metal ores are found in the earth's crust as sulfides or oxides.
Oxygen is the most plenteous element that is accessible in nature. It shapes 20.946% of air by volume and 46.6% of the world's mass generally as silicates and different compounds like carbonates, oxides, and sulfates.
The vast majority of the oxygen in the air is delivered by photosynthesis in plants. It additionally occurs as ozone. Sulfur is the sixteenth most inexhaustible element. Sulfur in its combined state is found in ores.
Sulphate Ores:
Includes gypsum, Epsom salt MgSO4 7H2O,CaSO4.2H2O, and barytes, BaSO4.
Sulphide Ores:
Includesgalena (PbS),zinc blende (ZnS), and copper pyrites (CuFeS2).
Sulphur can also be seen in many organic substances like mustard,eggs,seeds, onion, wool,garlic, and hair.
Selenium and tellurium are found in sulphides ores as metal selenides and tellurides.
Polonium is a radioactive element.
Electronic Configuration
Group 16 elements have 6 electrons in their valence shell and their general electronic configuration is ns2np4.
Element |
Electronic Configuration |
| Oxygen | [He] 2s2 2p4 |
| Sulphur | [Ne] 3s2 3p4 |
| Selenium | [Ar] 3d10 4s2 4p4 |
| Tellurium | [Kr] 4d10 5s2 5p4 |
| Polonium | [Xe] 4f14 5d10 6s2 64 |
Fig. 3: Electronic Configuration of Group 16 Elements
Atomic and Physical Properties and Their Trends
Atomic and Ionic Radii
The atomic and ionic radius increasesas we move from oxygen to polonium.
Ionization Enthalpy
Ionization enthalpy decreases with increase in size of the central atom as we move down.
Electron Gain Enthalpy
The electron gain enthalpy decreases with increase in size of the central atom moving down the group. Oxygen molecule has a less negative electron gain enthalpy than sulfur. This is on the grounds that, oxygen, because of its compressed nature encounter more repulsion between the electrons effectively present and the approaching electron. Because of these electron-electron repulsions, oxygen particle has lesser inclination than sulfur atom to acknowledge the additional electron.
Trend in Electronegativity
There is a progressive decline in electro negativity as we move down the group from oxygen to polonium due to increase in nuclear size. After fluorine, oxygen is the second most electronegative molecule.
Physical Properties of the Group 16 Elements
‘Oxygen' and "sulfur" are non-metals, "selenium" and "tellurium" are metalloids and "polonium" is a metal under typical conditions. Polonium is a radio-active element.
Allotropy
Each one of the element of group 16 display allotropy.
Oxygen has two allotropes:
-
Oxygen
-
Ozone
Sulphur exist as many allotropic forms but only two of them are stable:
-
Rhombic Sulphur
-
Monoclinic Sulphur
Selenium and Tellurium is found in both amorphous and crystalline forms.
The Melting and Boiling Points
As the atomic size increases from oxygen to tellurium, the melting and boiling points also increase.
The huge distinction between the melting and boiling points of oxygen and sulfur might be clarified on the premise that oxygen exists as a diatomic atom while sulfur exists as polyatomic particle.
Oxidation States
The group 16 elements have a configuration of ns2 np4 in their outer shell, they may accomplish noble gas configuration either by the gain of two electrons, framing M-2, or by sharing two electrons, in this manner shaping two covalent bonds. Thus, these elements indicate both negative and positive oxidation states. The regular oxidation states showed by the elements of group 16 incorporate -2, +2, +4, and + 6.
|
|
Element |
Oxidation States |
| 8O | Oxygen | -2,-1,+1,+2 |
| 16S | Sulphur | -2,+2,+4,+6 |
| 34Se | Selenium | -2,+2,+4,+6 |
| 52Te | Tellurium | -2,+2,+4,+6 |
| 84Po | Polonium | +2,+4 |
Oxygen shows high electro negativity. In all its metal oxides oxygen demonstrates a negative oxidation state of - 2. Despite - 2 oxidation states, oxygen illustrates - 1 oxidation state in peroxides and - 1/2 oxidation state in superoxides as can be seen in the diagram below:
Fig. 5: Different Species of Oxygen with their Oxidation States
Oxygen shows positive oxidation state just in its compounds with fluorine, since fluorine is more electro-negative than oxygen. It shows +2 oxidation state in OF2 and +1 in O2F2.
Remaining elements of the group, besides showing +2 oxidation states, also indicate +4 and +6 oxidation states due to the availability of d-orbitals in their particles.
Oxygen differs widely from the remaining elements of its group. This is because of its compact size, high electronegativity and the nonattendance of d-orbitals in the valence shell. The basic complexities are:
Atomicity:
Oxygen is gaseous and diatomic by nature, whereas the other elements of this group exist as solids.
Maximum Covalency:
Oxygen covalency is constrained to two, yet the covalency surpasses four in alternate elements because of the accessibility of d-orbitals in them.
Oxygen, because of its high electro-negativity, indicates negative oxidation state and does not demonstrate any positive oxidation state, aside from in OF2 and O2F2.
Hydrogen Bond Formation:
On account of its little size and high electro-negativity, oxygen shapes solid hydrogen bonds. Alternate elements in the group have relatively low electro-negativities, and don't shape hydrogen bonds.
Chemical Properties
The group sixteen elements react with hydrogen to frame hydrides of the sort H2E, where E could be any element oxygen, sulfur, selenium, tellurium or polonium.
Physical States of Hydrides
Water is an odorless and colorless liquid but the hydrides of the various elements of this group are poisonous gases which are colorless with disagreeable smells. The boiling point of these hydrides extraordinarily diminishes from water to hydrogen sulfide, and after that increases. Water has an anomalously high boiling point since its particles are bonded with each other by the hydrogen bonds in both its liquid as well as solid states.
Acidic Character of Hydrides
There is an expansion in acidic nature of hydrides from H2O to H2Te. The development in acidic character is a result of the decrease in the H-E bond separation enthalpy from H2O to H2Te. Except for water, the different hydrides go about as reducing agents. The reducing property of these hydride increment from H2S to H2Te.
Every one of the elements of group 16 reacts with oxygen to shape dioxides and trioxides. Both dioxides and trioxides are acidic in nature. Sulfur trioxide is the primary basic triode in this group. Sulfur trioxide at room temperature is a solid and exists in three specific structures - alpha, beta and gamma.
Elements of group 16 accommodate an enormous assortment of halides of the sort EX6, EX2, and EX4, where E is the element of group 16 elements and X is a halogen. Among all hexahalides, just hexafluoride are latent. They encounter sp3d2 hybridization, and along these lines, have octahedral geometry. SF6 is to an awesome degree stable. Among the tetra fluorides SeF4 is a liquid, SF4 is a gas, and TeF4 is a solid. Except for selenium, the different elements of this group shape dichlorides and dibromides.
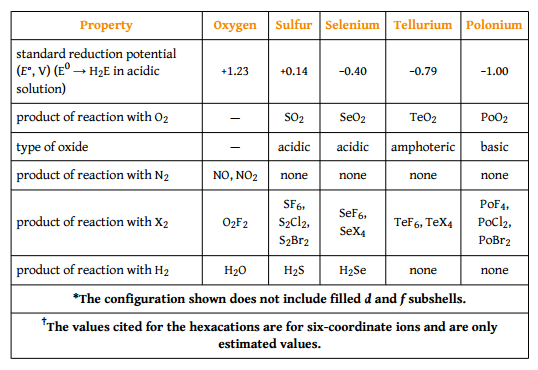
The following tables show the elected properties of group 16 elements in a tabulated manner:
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free