Hydrogen Chloride
Table of Content |
Discovery of Hydrogen Chloride
Fig. 1: structure of Hydrogen Chloride with bond length
In 1648, Glauber turned into the first person to manufacture hydrogen chloride by warming a blend of basic salt and sulphuric acid in concentrated form. In 1810, Davy demonstrated that it is a compound of chlorine and hydrogen.
Δ
NaCl + H2SO4 → NaHSO4 + HCl
Common salt Conc. Sulphuric Sodium Hydrogen
(Sodium Chloride) acid bisulphate Chloride
Manufacture of Hydrogen Chloride
Hydrogen Chloride is set up in the research facility by treating sodium chloride with concentrated sulphuric acid. The reaction blend, comprising of concentrated sulphuric acid and sodium chloride, is initially warmed to 420K.
420K
NaCl + H2SO4 → NaHSO4 + HCl
Common salt Conc. Sulphuric Sodium Hydrogen
(Sodium Chloride) acid bisulphate Chloride
Sodium bisulphate is acquired as a by result, which is insoluble. Subsequently, sodium bisulphate is further mixed with more sodium chloride and further warmed to a high temperature of around 823K to give dissolvable sodium sulfate and HCl gas.
823K
NaHSO4 + NaCl → Na2SO4 + HCl
Sodium bisulphate Sodium Chloride Sodium sulphate Hydrogen chloride
The HCl gas is dried by advancing it through concentrated sulphuric acid. HCl is not dried either over phosphorus pentoxide or brisk lime since it reacts with both of these compounds
Properties of HCl
Properties |
|
| Chemical Formula | CIH |
| Molar Mass | 36.46 g.mol-1 |
| Appearance | Colorless gas |
| Odor | Pungent |
| Density | 1.49 g L-1 |
| Melting Point | -114.22°C (-173.60°F; 158.93K) |
| Boiling Point | -85.05°C (-121.09°F; 188.10K) |
| Solubility in water | 823 g/L (0°C) |
| 720 g/L (20°C) | |
| 561 g/L (60°C) | |
| Solubility | Soluble in methanol, ethanol, ether |
Fig. 2: Properties of hydrogen chloride

Fig. 3: Structure of hydrogen chloride
-
Hydrogen Chloride is a vapid gas with an impactful pungent odor.
-
It melts to a colorless fluid at 189K and forms a white solid at 159K upon freezing.
-
It is exceedingly soluble in water.
-
An aqueous arrangement of Hydrogen Chloride is called hydrochloric acid.
-
An aqueous arrangement of Hydrogen Chloride experiences ionization to deliver hydronium particles and chloride particles.
-
This can be shown as below:
Fig. 4: An aqueous arrangement of hydrogen chloride experiences ionization to deliver hydronium particles and chloride particles
-
A higher value of dissociation constant (Ka) shows that hydrochloric acid is a strong acid.
-
HCl can dissociate very nearly 100 percent to create hydronium particles; it is viewed as a solid acid.
-
Hydrochloric acid reacts with metals and salts to shape chlorides
-
Example: It reacts with zinc to frame zinc chloride, and with sodium hydroxide to shape sodium chloride.
Fig. 5: Reaction of Hydrogen Chloride with zinc to frame zinc chloride, and with sodium hydroxide to shape sodium chloride
- Hydrochloric acid reacts with iron to shape ferrous chloride.
Fe + 2HCl FeCl2 +H2
Iron Ferrous chloride
- Hydrochloric acid reacts with ammonia to shape thick white vapour of ammonium chloride.
NH3 + HCl NH4Cl
Ammonium Ammonium Chloride
-
Noble metals break down in a concentrated arrangement of nitric acid and hydrochloric acid taken in the proportion of 1:3. This arrangement is known as aqua regia.
-
Hydrochloric acid breaks down salts of weaker acids, for example, sodium carbonate, sodium bicarbonate and sodium sulfite.
-
Hydrochloric acid breaks down sodium carbonate and sodium bicarbonate to sodium chloride, carbon dioxide, and water, while it deteriorates sodium sulfite to sodium chloride, sulfur dioxide, and water.
Sodium Carbonate
Na2CO3 + 2HCl → 2NaCl + CO2↑ + H2O
Sodium carbonate Sodium chloride carbon dioxide Water
NaHCO3 + HCl → NaCl + CO2↑ + H2O
Sodium bicarbonate Sodium chloride carbon dioxide Water
Sodium Sulphate
Na2SO3 + 2HCl → 2NaCl + SO2↑ + H2O
Sodium sulphite Sodium chloride Sulphur dioxide Water
Uses of Hydrochloric Acid
-
It is utilized as a part of the fabrication of chlorine, and chlorides like ammonium chloride. It is likewise utilized as a part of the fabrication of glucose from corn starch.
-
It is utilized as a research facility reagent and in medicines.
-
A saturated arrangement of zinc chloride in dilute hydrochloric acid is utilized to remove the contaminations on a metal surface before welding or electroplating.
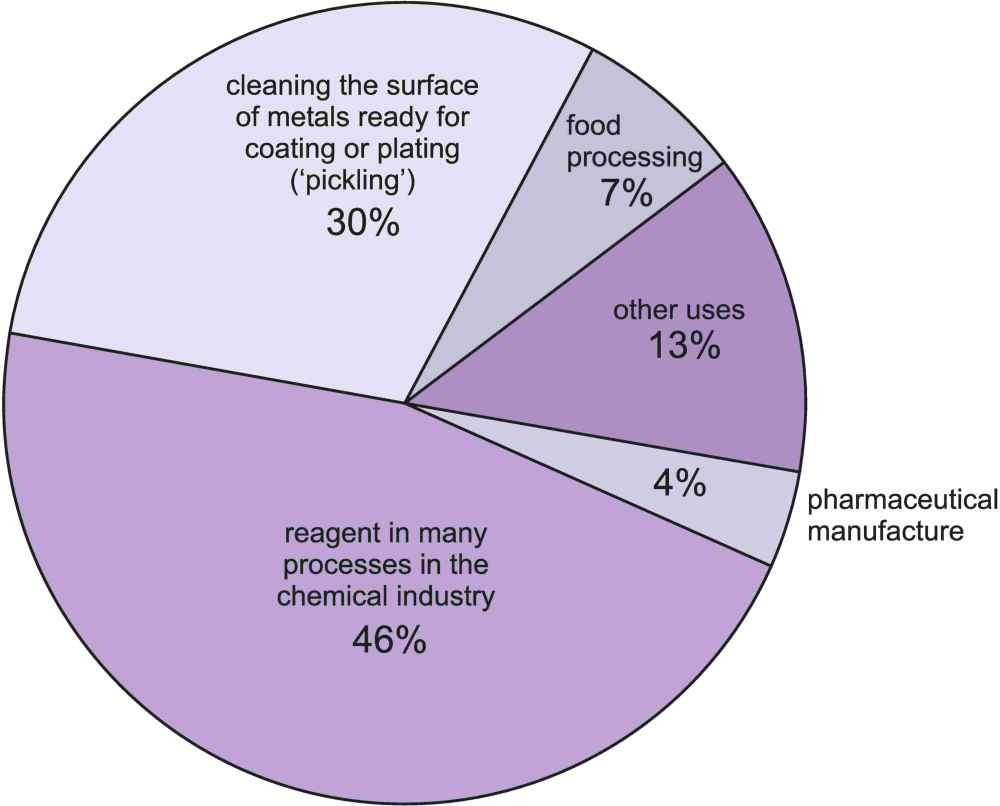
Fig. 6: A pie chart showing the principal uses of hydrogen chloride and hydrochloric acid.
Fig. 7: Effects of different HCl concentrations on health
Watch this Video for more referenceMore Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free