Important Trends and Anomalous Behaviour of Carbon
Fig. a: Structure and appearance of Carbon
Like the first individual from different groups, carbon likewise contrasts from whatever is left of the individuals from its own group. It is because of its small size, higher ionization enthalpy, higher electro negativity, and inaccessibility of d-orbitals.
-
In carbon, just s and p-orbitals are accessible for bonding and in this way; it can oblige just four sets of electrons around it. This would restrict the greatest covalence to four though different individuals can grow their covalence because of the availability of d-orbitals.
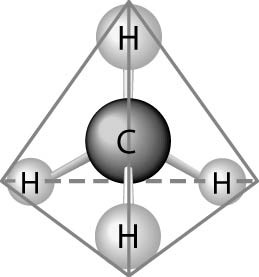
Fig. b: Tetrahedral Structure of Carbon
Fig. c: Electronic configuration of carbon showing 4 valence electrons
-
Carbon additionally has an extraordinary capacity to shape pp – pp multiple bonds with itself and with different molecules which have smaller size and high electro negativity. Couple of illustrations are: C = C, C° C, C = O, C = S and C° N. Heavier elements don't shape pp – pp bonds in light of the fact that their nuclear orbitals are too vast and diffuse to have viable overlapping.
-
Carbon atoms tend to interface with another through covalent bonds to shape chains and rings. This property is termed as catenation. This is on account of C – C bonds are extremely solid. This chain can be as large as to contain aggregate of 70-80 carbon. This offer ascent to extremely complex compounds having straight carbon chain, extended carbon chain and ring. The carbon compounds having just single bond are termed as saturated hydrocarbons while compounds having double or triple bond are termed as unsaturated hydrocarbons. As we move down the group the size increases and electro negativity diminishes and in this way, propensity to show catenation also decreases. This can be clearly observed from bond enthalpy values. The catenation order is C >> Si > Ge » Sn.
Fig. d: Long chains of carbon showing catenation
Lead does not indicate catenation
| Bond | Bond enthalpy kJ mol-1 |
| C – C | 348 |
| Si – Si | 297 |
| Ge – Ge | 260 |
| Sn – Sn | 240 |
- Because of the property of catenation and pp – pp bond development, carbon can indicate diverse allotropic structures as well.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More