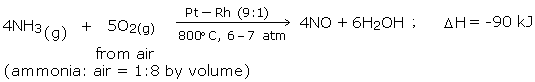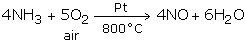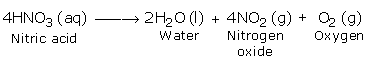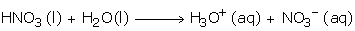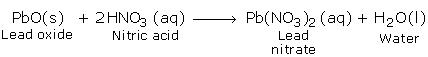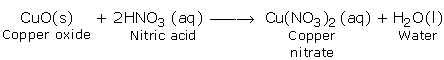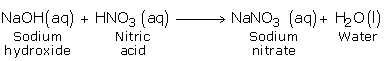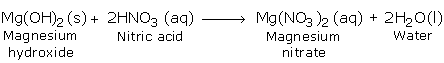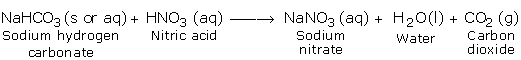Nitric Acid
Table of Content |
Introduction to Nitic Acid
The most imperative and helpful oxoacid of nitrogen is Nitric Acid. Its molecular formula is HNO3 and molar mass 63.01 g mol-1.
Fig. 1: Safety Data Sheet of Nitric Acid
Preparation of Nitric Acid
Laboratory Preparation
Nitric Acid can be manufactured by heating concentrated sulphuric acid with potassium nitrate. This process of heating is carried out in a glass retort and vapors of nitric acid are condensed in a receiver, which is further cooled by water. The reaction is:
Fig. 2: Laboratory Preparation of Nitric Acid
Industrial Preparation
On industrial scale, nitric acid is fabricated through the Ostwald's procedure - the process of catalytic oxidation of ammonia.
Ostwald's Process:
In this process the conversion of ammonia into nitric acid is done through the following steps:
Step1: Oxidation of Ammonia to Nitric Oxide
Ammonia is oxidized by air at 800°C in the presence of Pt catalyst to yield nitric oxide.
Step 2: Oxidation of NO to NO2
The nitric oxide is oxidized by air at temperature below 100°C, to yield nitrogen dioxide (NO2)
Step 3: Formation of Nitric Acid
Nitrogen dioxide by absorption in water is then converted to nitric acid, in the presence of air.
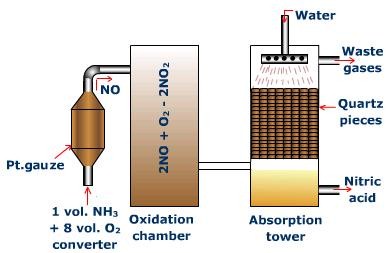
The Plant
The components and processes of the manufacturing plant for the production of nitric acid by the Ostwald's process are as follows:
Fig. 3: Ostwald's Process for the Manufacture of Nitric Acid
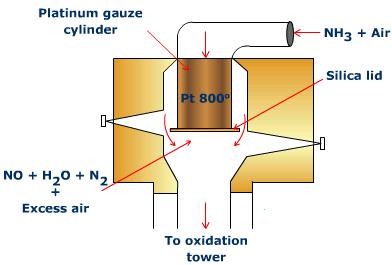
Converter
The converter is made of aluminum and fitted with platinum - rhodium gauze chamber (23 cm x 34 cm). The chamber is shut at the base with a silica top. The gauze is at first warmed to 800°C electrically. From that point no outside heating is required as oxidation of ammonia is an exothermic reaction. A blend of ammonia and clear air (volume proportion = (1:8) is then advanced through gauze from the top, and the products yielded leaves from the base. Each 1000 cm2 region of the gauze produces around 500 kg of nitric oxide, in every 24 hours.
Fig. 4: Converter for the Ostwald's Process
Oxidation tower
At the point when nitric oxide, containing nitrogen and some water vapors, leave the converter, they are cooled to around 100°C by advancing them through coolers. In the oxidation tower, nitric oxide is combined with more air and it gets changed over toNO2.
Absorption tower
NO2 is permitted to enter the ingestion tower from the lower end. The tower is stuffed with quartz pieces and water is sprinkled from the top. Here, NO2 is consumed into water in the presence of air to yield nitric acid.
Industrial nitric acid has brown shading because of the dissolved NO2. The system of gurgling dry air through warm industrial nitric acid is to release out the dissolved nitrogen dioxide so that the acid gets to be distinctly colorless.
Concentrating Nitric Acid
Aqueous nitric acid got by this strategy can be concentrated by refining to 68.5% by mass. A concentration of 98% acid can be accomplished by drying out with concentrated sulphuric acid. Anhydrous nitric acid can be gotten by refining of concentrated fluid nitric acid with phosphorus pentoxide (P2O5 or P4O10).
Fig. 5: Concentrating Nitric Acid
Structure of Nitric Acid
Nitric acid, a monobasic acid can be represented structurally as,
Fig. 6: Structure of Nitric Acid
Fig. 7: Chemical Structure of Nitric Acid
Nitric acid in gaseous form is a planar molecule. The bond angles and bond length in nitric acid molecule are shown in figure f.
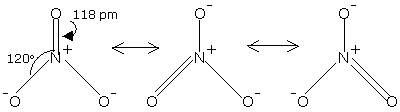
Structure of Nitrate Ion
The nitrate ion, NO3- is isoelectronic with carbonate ion, CO32-. It is likewise a planar particle. The nitrate particle can be represented by the resonance structures demonstrated as follows:
Fig. 8: Structure of Nitrate Ion
Physical Properties of Nitric Acid
-
Pure nitric acid is a colorless fuming fluid with an impactful smell. Pure acid or impure acid on standing creates yellow shading because of the presence of dissolved oxides of nitrogen (fundamentally NO2).
-
It is totally dissolvable in water and structures a steady boiling combination (120.5°C) with water, containing 68% (by mass) of nitric acid.
-
Pure acid has a thickness of 1.54 g/mL. The constant boiling combination has a thickness of 1.4 g/mL at 20°C.
-
Anhydrous nitric acid bubbles at 355.6 K (83.6°C) and develops a white solid on freezing at 231.4 K (- 41.7°C).
-
It has a corrosive activity on the skin and causes yellow rankles.
Chemical Properties of Nitric Acid
Stability
Nitric Acid in pure form is not exceptionally stable. Indeed, even at normal temperature, in the existence of daylight it experiences slight decay. As the temperature increases the rate of decay likewise increment. Upon strong heating it breaks down totally to give nitrogen dioxide, oxygen and water.
The nitrogen dioxide, which is reddish brown in color, may break down in the undecomposed acid to give it yellowish chestnut shade.
The above reaction additionally demonstrates that nitric oxide contains oxygen.
Activity
To show that Nitric Acid contains Oxygen
The apparatus is set up as shown in figure below:
Fig. 9: Thermal Decomposition of Nitric Acid
The ignition tube is heated unequivocally and concentrated nitric acid is permitted to stream into it. Because of the warmth, it breaks down, letting the comparing gasses free. The Reddish yellow exhaust of nitrogen dioxide, water vapor, and a colorless gas are acquired. Nitrogen dioxide and water vapor break down in the water while the colorless gas gets gathered in the gas jar. This gas revives a glowing splint, which ends up being oxygen.
Acidic Nature
Nitric Acid ionizes readily in water as follows and is a strong monobasic acid:
a) Reactions with Basic Oxides
b) Reaction with Bases (Hydroxides)
c) Reaction with Carbonates and Hydrogen Carbonates
d) Reaction with Metals
Nitric acid, as a rule, does not carry on as an acid with metals to shape the comparing salt and free hydrogen.
Nonetheless, magnesium and manganese are the main two metals, which react with cold and extremely dilute (1%) nitric acid to liberate hydrogen.
e) Reaction with Metallic Sulphites
Uses of Nitric Acid
The essential uses of nitric acid can be listed as follows:
- Nitric acid assumes a critical part in the fabricate of different items, for example:
-
Explosives like trinitrotoluene (T.N.T.), gun cotton, nitro glycerine, ammonal and so forth.
-
Fertilizers, for example, ammonium nitrate, calcium nitrate, and so on.
-
Nitrate salts, for example, calcium nitrate, ammonium nitrate, silver nitrate, and so on.
-
Dyes, scents, drugs and so on from coal tar items.
-
Sulphuric acid by Lead Chamber Process.
-
-
It is utilized as a part of the cleaning of silver, platinum, gold and so forth.
-
Nitric acid is utilized as a part of scratching designs on copper, bronze product, brass and so on.
-
It is utilized to make "aqua regia" for the disintegration of noble elements.
-
Aqua regia (Royal water in Latin) is an extremely solid acid. It is made by combining one section nitric acid and three sections hydrochloric acid. The acid was named by chemists since it can disintegrate the noble metals gold and platinum.
-
It is utilized as a research center reagent.
-
Nitric Acidum: The homeopathic medicine nitric ac. is a mineral which is arranged chemically utilizing extra care by mixing sodium nitrate and sulfuric acid.
Keep in mind: Ammonal is a blend of ammonium nitrate and aluminum powder.
The employments of nitric acid are abridged in the following figure:
Fig. Summary of uses of Nitric Acid
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free