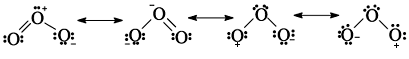Ozone
Table of Content |
General Characteristics and Occurrence of Ozone
Ozone is a triatomic allotropic type of oxygen that is unsteady. It is available in the climate for around 20 kilometers above ocean level.
It is framed from the oxygen that is present there, in the presence of ultraviolet light from the sun. Ozone is of imperative significance in shielding the world's surface from exposure to harmful ultraviolet radiations.
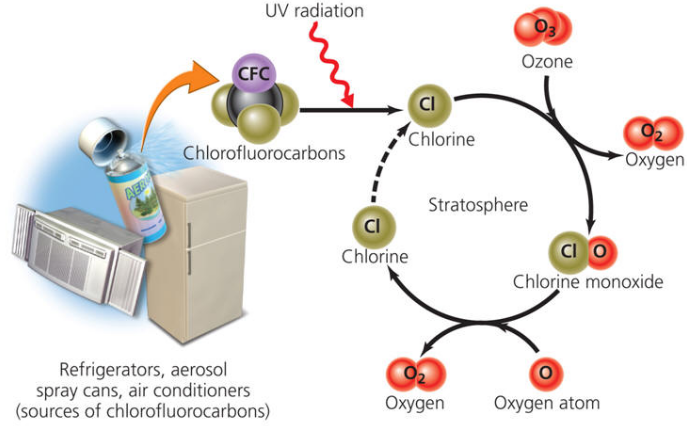
The utilization of chlorofluorocarbons in refrigerators and aerosols and their ensuing discharge into the air is responsible for making gaps in the ozone layer over the Antarctic and Arctic areas. It is expected that this will permit intemperate UV light to achieve the earth, which can bring about skin malignancy in people.
Fig. 1: Above diagram shows how CFCs affect Ozone layer
The oxides of nitrogen, specifically nitric oxide, react very rapidly with ozone to give nitrogen dioxide and oxygen. Hence, the oxides of nitrogen discharged from the fumes frameworks of supersonic fly planes gradually deplete the ozone layer.
Fig. 2: Ozone depletion
Preparation of Ozone
Ozone is set up by passing a silent electric discharge through dry, unadulterated, and cold oxygen in an extraordinary device called the ozoniser. This way concentration of approximately 10% of ozone can be obtained. The ozone formation is an endothermic process; it must be completed at high temperature. This is the reason; it is set up by method of silent electric discharge.
Fig. 3: Preparation of Ozone by Silent Electric Discharge method
If further high concentrations or pure ozone is to be prepared, it can be obtained by fractional liquefaction of an oxygen-ozone mixture.
Physical Properties of Ozone
Ozone is a light blue gas with a characteristic fishy smell. At - 1200C, it condenses as a dull blue fluid, which on further cooling, gets hardened to violet dark crystals. Ozone is thermodynamically unsteady and disintegrates to oxygen. The decay is exothermic and is catalyzed by numerous materials. High concentrations of ozone are hazardously dangerous.
Oxidizing Action of Ozone
Ozone is considered to be a very strong oxidizing agent because of the ease with which it liberates atoms of nascent oxygen. It oxidizes,
-
Lead sulphide to lead sulphate.
4O3 + PbS → 4O2 + PbSO4
-
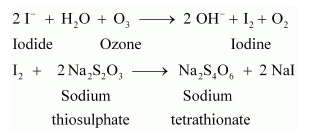
Iodide ions to iodine
2KI + H2O + O3 → 2KOH + I2 + O2
This reaction is used in quantitative estimation of ozone. When ozone is made to react with potassium iodide solution buffered with a borate buffer (pH 9.2), iodine is liberated. This liberated iodine can be titrated against a standard solution of sodium thiosulphate using starch as an indicator. The reactions involved in the process are given below:
- Nitrogen dioxide to dinitrogen pentoxide
2NO2 + O3 → N2O5 + O2
Structure of Ozone
The ozone atom is symmetrical and exhibits a bond angle of about 1170.
 |
 |
|
Fig. 4: Bond Angle of ozone |
Fig. 5: The bond lengths between the central oxygen and the other two oxygens are identical |
Since Ozone is unstable, it is actually a resonance hybrid of the canonical forms.
Fig. 6: Structure of the ozone molecule as a resonance hybrid of the four canonical forms
Uses of Ozone
-
Ozone is used as an antiseptic.
-
Ozone is moreover used as a disinfectant. For Example, it is used in filtration of drinking water.
-
It is used as a delicate dying agent for fading oils, starch, ivory, wax, flour, and delicate textures
-
It is used as a part of the produce of potassium permanganate from potassium manganate.
-
It is moreover used as a part of the creation of manufactured camphor and artificial silk.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free