Phosphine
Table of Content |
Occurrence and Discovery of Phosphine
 Phosphine is a chemical which has a place with the group of organophosphorus compounds. This compound was initially acquired by Philippe Gengembre in the year 1783. He prepared Phosphine by providing heat to phosphorous in an aqueous arrangement of potassium carbonate. The chemical formula for Phosphine is PH3.The concentration of this compound changes in the environment. It assumes an essential part in phosphorous biochemical cycle.
Phosphine is a chemical which has a place with the group of organophosphorus compounds. This compound was initially acquired by Philippe Gengembre in the year 1783. He prepared Phosphine by providing heat to phosphorous in an aqueous arrangement of potassium carbonate. The chemical formula for Phosphine is PH3.The concentration of this compound changes in the environment. It assumes an essential part in phosphorous biochemical cycle.
Preparation
(i) From Phosphide
By the reaction of phosphides with water
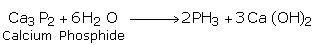

Upon heating Phosphorus acid decomposes to give a pure sample of phosphine.
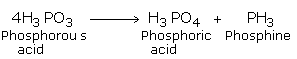
Structure of Phosphine
Fig. 1: Electron dot Structure of Phosphine
The electronic structure of phosphine is like ammonia in that it has a pyramidal structure. The bond angle H-P-H, in any case, is = 93° (e.g., NH3 =107o)
Ammonia has pyramidal geometry with a bond angle of 107.80. So also, Phosphine is likewise anticipated that would have pyramidal geometry with about the same or comparative bond angle.
In any case, the bond angle in Phosphine is just 93.5°. Phosphorus has less electro-negativity than nitrogen.
Fig. 2: Bond Angle and Bond Length of Phosphine
The electron thickness around the central atom-phosphorus is less concentrated when contrasted with nitrogen present in ammonia. Subsequently, the lone pair causes significantly more noteworthy contortion in Phosphine. Accordingly, the bond angle in Phosphine declines to 93.5°.
Physical and Chemical Properties of Phosphine
-
It is a colorless gas and has a spoiled fish smell.
-
It is exceedingly noxious gas.
-
PH3 is sparingly dissolvable in water and dissolvable in natural solvents.
-
PH3 goes about as a Lewis base by giving its lone pair of electrons when it reacts with hydrogen iodide.
-
Under typical conditions, it is a non-ignitable gas, yet when warmed it bursts into flames which bring about the formation of phosphoric acid.
PH3 + 2O2 → 150c H3PO4
- It explodes violently when exposed to oxidizing agents.
Uses of Phosphine
-
In semiconductor industries, it is utilized in parts as a dopant.
-
PH3 is utilized as a part of Holme's signal because of its property of sudden ignition. Compartments containing calcium carbide and calcium phosphide are penetrated and tossed in the ocean when the gasses developed smolder and serve as a signal. It is likewise utilized as a part of smoke screens. Containers which have a punctured base and a gap at the top are recorded with calcium phosphide and calcium carbide. These are tossed into the ocean. Water enters the containers through the base and reacts to give acetylene and phosphine. Phosphine gets ignited suddenly as it interacts with air and furthermore ignites acetylene. Along these lines, a splendid red fire is delivered which is joined by enormous smoke because of the smoldering of phosphine. This serves as a signal to the approaching boats.
-
Phosphine fumigants are generally utilized on homesteads to control bug, rat and rabbit pervasion in a wide range of stored grains. The level at which people recognize the smell of phosphine (scent edge) does not give adequate cautioning of perilous concentrations. It is transported as compressed liquefied gas. A few solids (phosphides) discharge phosphine gas. At the point when phosphine lethality is suspected, however phosphine exposure has not happened, ingestion or transdermal defilement from phosphides ought to be considered.
Phosphine Gas Effects
Introduction to even little measures of phosphine can bring about cerebral pain, dizziness, sickness, regurgitating, loose bowels, laziness, chest tightness and cough. More genuine presentation can bring about coma, shock, convulsions, liver and kidney damage and irregular heartbeat.
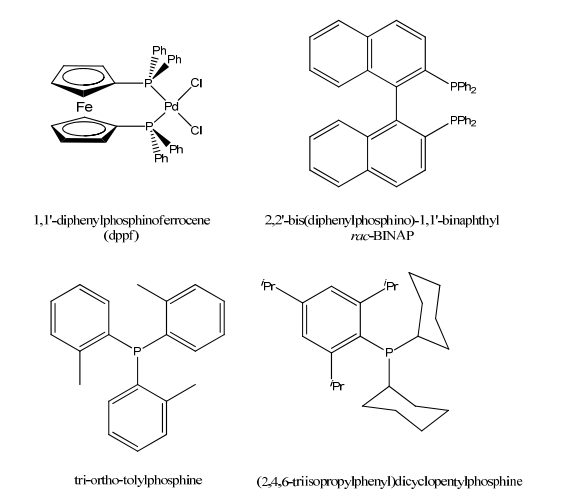
Phosphine Ligands
-
Tertiary phosphines, PR3, are a vital class of ligands in light of the fact that their electronic and steric properties can be changed in an orderly and unsurprising path over a wide range by shifting the R group(s).
-
Tertiary phosphines likewise stabilize an extraordinarily wide assortment of metal complexes of interest to the organometallic scientific expert as their phosphine complexes (R3P) nM−L.
-
Phosphines are more normally spectator as opposed to performer ligands.
Fig. 3: Phosphine Ligands
-
The range and many-sided quality of phosphine ligands has expanded significantly as of late because of the advancement of C-X coupling reactions, For Example, Buchwald-Hartwig reaction.
Fig. 4: Phosphine Ligands
-
Like NR3, phosphines have a lone pair on the focal particle that can be given to a metal.
-
Unlike NR3, they are likewise π-acids, to a degree that relies on upon the way of the R groups display on the PR3 ligand.
-
For alkyl phosphines, the π acidity is very weak; aryl, dialkylamino, and alkoxy groups are progressively more viable in advancing π acidity.
-
In the outrageous instance of PF3, the π acidity gets to be as incredible as that found for CO.
-
In the instance of CO the π* orbital acknowledges electrons from the metal.
-
The σ* orbitals of the P−R bonds assume the part of acceptor in PR3.
Watch this Video for more reference
More Readings