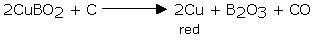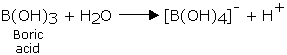Some Important Compounds of Boron
Table of Content |
The Element Boron
Atomic Number: 5
Atomic Weight: 10.811
Melting Point: 2348 K (2075°C or 3767°F)
Boiling Point: 4273 K (4000°C or 7232°F)
Density: 2.37 grams per cubic centimeter
Phase at Room Temperature: Solid
Element Classification: Semi-metal
Period Number: 2 Group Number: 13 Group Name: Group 13 (IIIA)
Introduction to the various compounds formed by Boron and their Applications
The most imperative of boron compounds is sodium borate (Na2B4O7); utilized as a part of the fabrication of borosilicate glass, glass fiber protection, and material glass fiber. The introduction of sodium borate to glass makes it less demanding to work while it is liquid. The last glass is not assaulted by acids or water, is extremely solid, and opposes heat shock. Imperviousness to heat shock implies the glass can be warmed and cooled rapidly without breaking. The Pyrex glass utilized as a part of kitchenware and science research facilities is a type of borosilicate glass. Superb optical glass, similar to that utilized as a part of telescopes, is likewise produced using borosilicate glass.
Glass fiber insulation is likewise produced using borosilicate glass by driving it through thin openings. The glass turns out as a thin fiber and is then spun into insulation. These strands trap air. Since neither the borosilicate strands nor air is a decent conductor of warmth, it makes magnificent insulation. A great part of the insulation utilized as a part of private homes, office structures, and different structures is made of borosilicate filaments.
Filaments produced using borosilicate glass is additionally utilized as a part of making fabrics. Borosilicate strands are mixed with other artificial filaments to make sturdy texture for vehicle seat covers and other long-wear approaches.
Boron likewise shapes imperative compounds with two different components, carbon and nitrogen. Boron carbide (B4C) and boron nitride (BN) are essential compounds as a result of their hardness. Indeed, boron nitride might be the hardest known substance. Both compounds have high melting points: for boron carbide 2,350°C (4,300°F) and for boron nitride more than 3,000°C (5,400°F).
The above-mentioned properties make boron carbide and boron nitride valuable as abrasives and refractories. A grating is a fine material used to polish or grind different materials. A recalcitrant material is one that can withstand high temperatures by reflecting warmth. Refractory materials line microwaves to keep up high temperatures.
Research recommends that an absence of boron may prompt to joint pain and different issue of the skeleton.
Boron carbide and boron nitride are utilized as a part of rapid devices, military flying machine and shuttle, specialized heat- resistant filaments and warm shields. They additionally are found in cream make-ups, face powders, and lipsticks.
Little measures of boron compounds are additionally used to control the development of weeds in farming, and as bug sprays, fertilizers, and fire retardants. A fire resistant is a material that keeps another material from catching the flame and further burning with an open fire.
Borax (Na2B4O7.10H2O)
Borax is found occurring naturally as tincal (that contains around 55% borax) in certain inland pools of Tibet, India, and California (U.S.A.).
Manufacture of Borax:
From Tincal
It is acquired from tincal by extricating it with water, further concentrating the solution and abandoning it for crystallization, when gems of borax, Na2B4O7.10H2O segregate.
From other Minerals
Borax can likewise be set up from minerals, for example, boracite, boronatrocalcite, and colemanite. These minerals are powdered and then boiled with sodium carbonate arrangement:
Borax is solidified from the filtrate. Sodium metaborate, found in the mother alcohol, is changed over into borax by advancing carbon dioxide through it.
Two important hydrates of borax are:
-
Decahydrate or monoclinic borax, Na2B4O7.10H2O.
-
Pentahydrate, Na2B4O7.5H2O.
The pentahydrate is acquired when the solution is solidified at over 60°C, while the monoclinic type is acquired when crystallization is completed beneath 60°C. Both theassortments of Borax, after heating prompts to the formation of anhydrous borax.
Properties of Borax
Borax is a lackluster, crystalline solid sparingly solvent in icy water, however, breaks up promptly in high-temperature water. It frames two imperative hydrates: octahedral borax Na2B4O7.5H2O and monoclinic borax Na2B4O7.10H2O.s
Basic Nature
Borax is marginally hydrolyzed in solution. As boric acid is a feeble acid, the solution is alkaline in nature.
Action of Acids
Borax responds with HCl or H2SO4 to frame boric acid. On cooling, white pieces of boric acid are acquired.
Action of heat
At the point when powdered borax is warmed emphatically in a bunsen-fire, it loses water of crystallization and structures dry, colorless, transparent glass-like bead, structured up of boric anhydride and sodium metaborate.
Borax - Bead test
Boric anhydride undergoes reaction with a few salts of metals such as, Ni2+, Co2+, Cr3+, Cu2+, Mn2+ etc. resulting in the formation of colored metaborates. The shade of the metaborates is utilized to recognize the metallic particle (cation) in the salt.
Take a little amount of borax on a little circle of platinum wire to play out the test. At the point when this is warmed in a fire, till a transparent bead is framed. The hot transparent bead is touched with the salt when a couple of particles stick to it. The bead (with the sticking salt) is then warmed in an oxidizing fire (external zone) and afterward in a reducing fire (radiant zone). From the shade of the bead when hot and when cool, the essential radical present in the salt can be distinguished.
Certain metaborates get reduced to form the free metal in a reducing fire. For metals with variable oxidation states, the metaborate changes to lower valence state. For instance, with copper sulfate, the borax bead test gives,
In oxidizing flame:
In reducing flame:
Nickel salts give chestnut colored bead; cobalt gives blue shaded bead, while chromium salt gives green colored bead.
Structure of Borax
In borax, two boron iotas are in triangular geometry and two boron particles are in tetrahedral geometry. The particle is [B4O5(OH)4]2-and the ![B4O5(OH)4]2− Anion Structure In Borax B4O5(OH)4]2− Anion Structure In Borax](https://files.askiitians.com/cdn1/images/2017210-114619694-1844-12-some-important-compounds-of-boron.png) left over eight water atoms are related with the two sodium particles. Henceforth, borax contains tetranuclear units [B4O5(OH)4]2-, and is formulated as Na2(B4O5(OH)4].8H2O.
left over eight water atoms are related with the two sodium particles. Henceforth, borax contains tetranuclear units [B4O5(OH)4]2-, and is formulated as Na2(B4O5(OH)4].8H2O.
For the most part borax is depicted as Na2B4O7•10H2O, it contains [B4O5(OH)4]2− units so it is for the better defined as Na2[B4O5(OH)4]•8H2O. In this structure, there are two sp2 hybridized boron particles with triangular geometry and two boron molecules in tetrahedral geometry (sp3 hybridization), with each conveying a formal charge of −1.
Uses of Borax
-
For producing enamels, ceramics, coats, optical glass, cleansers and drying oils.
-
For hardening the wicks of candles.
-
In the production of washing powders and cleansers as a fabric softener.
-
A scientific reagent e.g., in borax bead test.
-
As a germicide.
Boric Acid (H3BO3)
 Boron trioxide with various measures of water gives a few boric acids. The most imperative of these is orthoboric acid (boric acid), H3BO3.
Boron trioxide with various measures of water gives a few boric acids. The most imperative of these is orthoboric acid (boric acid), H3BO3.
Preparation of Boric Acid:
-
From borax
Boric acid is acquired by the activity of hydrochloric acid or sulphuric acid on borax. The reaction mixture on cooling gives white pieces of boric acid.
-
By the hydrolysis of boron compounds
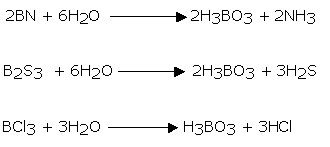
Boric acid may likewise be gotten by the hydrolysis of most boron compounds like nitrides, sulfides, halides and so forth.
Properties of Boric Acid
-
Boric acid is a delicate, white crystalline substance, and lathery to touch.
-
Boric acid is tolerably solvent in cool water, however genuinely dissolvable in boiling point water.
Action of heat
On warming boric acid breaks down to frame metaboric acid at 375 K, tetraboric acid at 435 K and boron trioxide at red heat temperature.
Acidic Character
Boric acid behaves as a weak monobasic acid, (Ka = 1x10-9). Upon accepting a hydroxyl ion it behaves as a Lewis acid,
Boric acid forms salts upon reaction with strong alkalies known as metaborates.
Structure of Boric Acid
 Boric acid consists of planar BO33-units bound together through hydrogen bonds shaping a trigonal planar layer structure. Hydrogen iotas go about as extension between two oxygen molecules of various BO33-units.
Boric acid consists of planar BO33-units bound together through hydrogen bonds shaping a trigonal planar layer structure. Hydrogen iotas go about as extension between two oxygen molecules of various BO33-units.
Uses of Boric Acid
-
It utilized as a part of the fabrication of ceramics coats and enamels.
-
As a food preservative in nourishment industry.
-
For manufacturing borosilicate glass.
-
In medicines as eyewash.
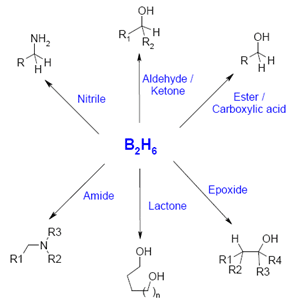
Diboranes (B2H6)
 Diborane (B2H6) is another fundamental compound of boron. Boranes are a class of compounds containing just boron and hydrogen. Diborane is dry, exceedingly unsafe and to an extraordinary degree responsive.
Diborane (B2H6) is another fundamental compound of boron. Boranes are a class of compounds containing just boron and hydrogen. Diborane is dry, exceedingly unsafe and to an extraordinary degree responsive.
Diborane is acquired by the oxidization of sodium borohydride with iodine.
The reaction between diborane in oxygen is to an incredible degree exothermic, releasing 1976 kJ of energy for each mole of diborane.
Boranes were once considered for use as solid fuels for rockets by virtue of their exothermic reaction with oxygen.
Diborane is an electron deficient atom. The two boron particles and the four terminal hydrogen particles of the molecule are all on a comparable  plane. These four terminal B - H bonds are steady 2-centred 2 electron bonds.
plane. These four terminal B - H bonds are steady 2-centred 2 electron bonds.
The traverse hydrogen particles lie above and underneath this plane. The two augmentations B-H-B bonds are unpredictable three centred two electron bonds. The boron atoms in diborane encounter sp³ hybridisation. The covering of a purge sp³ hybrid orbital of one boron particle and sp³ hybrid orbital of another boron atom containing one electron with the pure s - orbital of interfacing hydrogen containing one electron achieves the "banana bond". Likewise, another banana bond is surrounded on inverse side.
Therefore the two banana bonds shaped lie above and underneath the plane of the boron particles. The left over two sp³ hybrid orbitals of every boron iota cover with terminal hydrogen iotas to frame ordinary sigma bonds brings about the development of four terminal hydrogen bonds.
Reactions of Diborane
With ammonia
Boron Nitride (BN)n
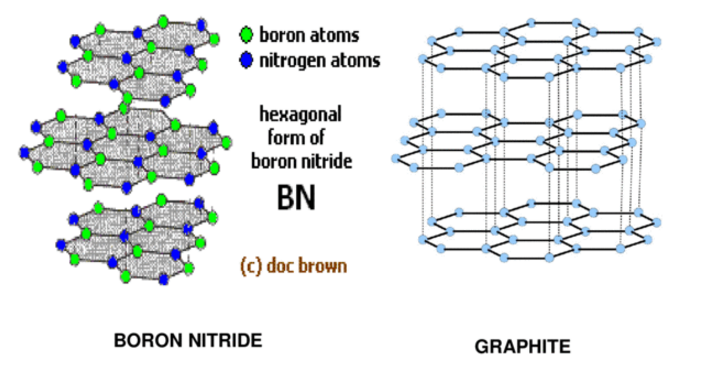
Boron nitride is a chemical compound having the chemical formula BN, comprising of equivalent quantities of boron and nitrogen iotas. BN is isoelectronic to a comparably organized carbon cross section and consequently exists in different crystalline structures. The hexagonal frame relating to graphite is the most steady and gentlest among BN polymorphs and is therefore utilized as a grease and an added substance to cosmetic items. The cubic assortment undifferentiated from precious stone is called c-BN. Boron nitride is not found in nature and is therefore created artificially from boric acid or boron trioxide.
The underlying item is amorphous BN powder, which is changed over to crystalline h-BN by heating in a nitrogen stream at temperatures over 1500 °C. As a result of incredible chemical and thermal stability, boron nitride ceramics are generally utilized as parts of high-temperature gear. Boron nitride has an awesome potential in nanotechnology. Nanotubes of BN can be delivered that have a structure like that of carbon nanotubes, a BN nanotube is an electrical encasing. Like other BN shapes, BN nanotubes are more thermally and chemically stable than carbon nanotubes which favor them for a few applications.
Preparation of Boron Nitride
- By heating boron in nitrogen, NO or NH3
2B+N2 → 2BN
5B + 3NO → 3BN + B2O3
2B + 3NH3 → 2BN + 3H2
- Anhydrous borax is heated with dry NH4Cl to read in a platinum crucible
Na2B4O7 + 2NH4Cl → 2BN + 2NaCl + B2O3 + 4H2O
- B2H6 reacts with excess of NH3 at high temp, BN is obtained
Watch this Video for more reference
More Readings