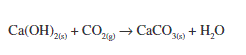Some Important Compounds of Calcium
Table of Content |
Fig. 1. General properties of calcium
The important compounds of calcium/common compounds of calcium are as follows:
Calcium Oxide (CaO)
-
Calcium Oxide is also known as Quick Lime.
-
It is a white, amorphous solid.
-
Heating of calcium carbonate forms calcium oxide and carbon-dioxide.
Fig. 2. Formation of calcium hydroxide from calcium oxide
- Calcium oxide on hydrolysis forms calcium hydroxide.
-
Calcium carbonate on decomposition forms calcium oxide and carbon-dioxide.
-
Calcium oxide on reaction with carbon-dioxide forms calcium carbonate.
-
It is an important ingredient for preparing cement.
-
Caustic soda and calcium oxide is used in the manufacturing of sodium carbonate.
Calcium Hydroxide (Ca(OH2))
-
Also, known as Slaked Lime, Ca(OH)2.
-
Adding water to calcium oxide forms calcium hydroxide.
-
It is slightly soluble in water.
-
Passing carbon-dioxide through calcium hydroxide forms calcium carbonate and water.
-
Diluted solution of calcium hydroxide is known as Lime Water.
-
A suspension of calcium hydroxide particles giving it a milky aspect, is known as Milk of Lime.
-
Passing of carbon-dioxide through calcium hydroxide forms calcium carbonate. But when excess of carbon-dioxide is passed through, calcium hydrogen carbonate is formed.
-
Passing chlorine through calcium hydroxide forms hypochlorite. Hypochlorite is one of the constituent of bleaching powder.
-
It is used to prepare building material mortar.
-
It has disinfectant property.
-
Calcium hydroxide is also used in tanning industry, glass making etc.
Calcium Carbonate (CaCO3)
-
Calcium carbonate occurs in different forms such as limestone, marble, chalk etc.
-
When carbon-dioxide is passed through calcium hydroxide, calcium carbonate is formed.
-
Reaction of sodium carbonate with calcium chloride also forms calcium carbonate.
-
Calcium carbonate is insoluble in water.
-
Decomposition of calcium carbonate forms quick lime, that is, calcium oxide and carbon-dioxide.
-
Marbles made up of calcium carbonates is used as building material.
-
Used for the extraction of the iron.
-
Used in the manufacturing of the paper.
-
Calcium carbonate is also used to treat acidity.
-
It is also a constituent of toothpaste, chewing gum etc.
Calcium Sulpahte (CaSO4)
It is commonly known as Plaster of Paris
Fig. 3. Formation of gypsum
-
After heating gypsum for a long time, it forms anhydrous calcium suphate. Anhydrous calcium suphate is known as “Dead Burnt Plaster”.
-
It is used in building industries.
-
It is also used for fixing bone parts after fracture.
-
Used for making statues.
Cement
-
It is also known as Portland Cement.
-
It is the most commonly used building material.
-
It is made up of silicon dioxide, calcium oxide, aluminum, iron, and magnesium.
-
Limestone and clay are two raw materials for cement formation.
-
Another important ingredient of cement is dicalcium silicate, tricalcium silicate and, tricalcium aluminate.
-
It is the most common material used during plastering.
-
It is used in construction of dams, bridges, and buildings.
Frequently Asked Questions(FAQs)
Q1. What is the state of calcium at room temperature?
Sol. At room temperature, calcium exists in the solid form.
Q2. Is calcium a solid or a liquid?
Sol. Calcium exists naturally as solid.
Q3. What are the uses of the element calcium? OR What is calcium used for?
Sol. Calcium is used as a reducing agent during preparation of thorium and uranium.
It is used in making alloys with aluminum, copper, lead etc. Calcium is essential for bones and teeth.
Q4. Isotopes of calcium?
Sol. Calcium has 24 isotopes. Out of these, five stable isotopes are as follows-
40Ca, 42Ca, 43Ca, 44Ca and 46Ca.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free