Sulphur: Allotropic Forms
Table of Content |
Introduction to the Element - Sulphur
Sulphur, with symbol S, is a chemical element with atomic number 16 and is additionally termed spelt sulfur. It is promptly accessible at room temperature as a splendid yellow crystalline solid and is a non- metal.
Fig. 1: Sulfur symbol, atomic number, symbol and mass number
Fig. 2: Position of sulfur in periodic table
Sources of Sulphur
Sulfur can be found from the accompanying sources:
(1) Extraction from underneath the earth outside layer - this is the most imperative source.
(2) From natural gas - this is the second most imperative source. The characteristic gas is found in a place called Lacq, in southern France.
(3) From different procedures - example, as a by-result of the refining of petroleum and decontamination of crude coal gas.
Properties of Sulphur
Sulphur exhibits the following properties:
Physical Properties
-
Sulfur is a yellow solid and is insoluble in water yet soluble in toluene (methyl benzene) and carbon disulphide.
-
Sulfur is a non-metal and therefore a poor channel of electricity and heat.
-
The boiling point of sulfur is 444oC. At the point when sulfur vapor is consolidated, a fine powder, which shapes a pattern resembling flower is gotten - this is called 'Flower of Sulfur’.
Chemical Properties
(1) Most metals and non-metals react specifically with sulfur
Example: without air, sulfur consolidates straightforwardly with most metals to frame sulphides when heated
Fe(s) + S(s) → FeS(s)
Zn(s) + S(s) → ZnS(s)
Pb(s) + S(s) → PbS(s)
2Cu(s) + S(s) → Cu2S(s)
Note: the reactive elements, similar to Na and K may react with sulfur suddenly (without heating).
(2) Sulfur smolders in abundance of air with a splendid blue fire, to form sulfur (IV) oxide and a little amount of sulfur (VI) oxide
O2(g) + S(s) → SO2(g)
(3) Sulfur joins gradually at high temperature with hydrogen, to form hydrogen sulfide
H2(g) + S(s) → H2S(g)
(4) Sulphur vapor joins with hot coke to form a fluid - carbon disulphide
C(s) + S(g) → CS2(l)
(5) Activity of Oxidizing Acids on Sulphur
Note:
-
With hot conc. HNO3 using bromine as catalyst, sulphur is oxidized to tetraoxosulphate(VI) acid.
6HNO3(aq) + S(s) → H2SO4(aq) + 6NO2(g) + 2H2O(l)
-
With hot concentrated H2SO4, sulphur is oxidized to sulphur(IV) oxide, SO2, while the acid is reduced to SO2.
2H2SO4(aq) + S(s) → 2H2O(l) + 3SO2(g)
-
With dilute acids and with conc. HCl - no reaction with sulphur.
(6) Action of Hot Concentrated Alkalis
With hot conc. alkalis, sulfur shapes a blend of sulfides and sulfate (IV). In the presence of excess sulphurPolysulphide and thiosulphate(VI) are formed.
3S + 6OH- → 2S2- + SO32- + 3H2O
With excess sulphur, SO32- + S → S2O32- (thiosulphate(VI))
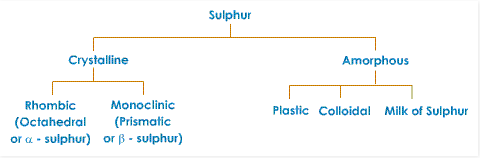
Allotropes of Sulphur
Fig. 4: Allotropes of Sulfur
Sulfur exists in various structures (in the same physical state). There are two critical crystalline structures - rhombic or octahedral (α - sulfur), and monoclinic sulfur (β - sulfur).
Note: one imperative distinction between these two types of crystalline sulfur is temperature.
Rhombic sulfur takes shape at a temperature beneath 96oC, while monoclinic sulfur solidifies at a temperature over 96oC.
Fig. 5: Allotropes of sulfur at transition temperature
The temperature, 96oC is known as the transitional temperature between the two structures.
Another allotrope of sulfur is polymeric sulfur (S8). It is an eight-part ring particle. At a temperature of 160oC or higher, the sulfur particle is energized and inevitably ruptures.
It is insoluble in organic media, synthetic and natural rubber, and also in carbon disulfide.
A proof of the above being allotropes is that each one can be changed over to the next without an adjustment in mass. In the event that equivalent masses of the allotropes are changed over into a given compound, illustration, H2S, similar masses of items are obtained.
A few different changes of sulfur-containing 6-20 sulfur particles for every ring have been synthesized artificially. In cyclo S6, the ring takes over the chain shape measurements.
Below is a summary of the properties of both rhombic and monoclinic sulphur.
Rhombic Sulphur
-
Yellow, translucent crystals
-
Melting point of 114oC
-
Density of 2.08 gcm3
-
Stable at temperatures below 96oC
Fig. 6: Preparation of rhombic sulfur
Fig. 7: Crystals of rhombic sulfur
Monoclinic Sulphur
-
Transparent, amber crystals
-
Melting point of 119oC
-
Density of 1.98gcm3
-
Unstable at temperatures below 96oC, reverting to rhombic form
Fig. 8: Preparation of monoclinic sulfur
Fig. 9: Crystals of monoclinic sulfur
Note: The temperature at which one allotrope of an element changes to another allotrope of the same elementis called Transition Temperature, e.g. at 96oC or above, rhombic sulfur changes to kaleidoscopic or prismatic sulphur. At 96oC or beneath, kaleidoscopic or prismatic sulfur changes to rhombic sulfur.
These allotropes that can change their configuration from one form to another by some changes in temperature are known as Enantiotropic Allotropes.
Plastic Sulphur
On heating sulfur, till nearly the boiling point and all of a sudden cooling it by filling cold water a thick mass is shaped. This sudden cooling does not permit adequate time to the atoms to adjust themselves to shape monoclinic or rhombic types of sulfur. Thus, the atoms frame an intertwined mass, comprising of both rhombic and monoclinic assortments of sulfur. This is called Plastic Sulphur.
This sort of sulfur is a dark brown or even dark, sticky substance. It is versatile. It has no sharp melting point. It doesn't break up in carbon disulfide. On standing, it gradually changes to the rhombic structures, as it picks up the eight molecule ring structure.
Fig. 10: Plastic Sulfur
Colloidal Sulphur
This sort of sulfur is set up when hydrogen sulfide is passed through a saturated and cooled solution of sulfur dioxide in water, or by including a solution of alcohol and sulfur in the water. Colloidal sulfur is solvent in carbon disulfide. It is utilized as a part of medicines.
Fig. 11: Colloidal Sulfur
Milk of Sulphur
Milk of sulfur is set up by the activity of weaken hydrochloric acid on ammonium sulfide. Milk of sulfur is likewise arranged by boiling move sulfur with calcium hydroxide (aqueous solution). This mixture is the filtered and weaken hydrochloric acid is added to the filtrate to get milk of sulfur.
Milk of sulfur is non-crystalline and white in shading. It is soluble in carbon disulphide. At the point when it is heated, it changes to the conventional yellow assortment of sulfurwhich is used as a part of medicines.
Uses of Sulphur
The accompanying is a portion of the ways sulfur is utilized:
-
For the development of specific sorts of fungus in vines.
-
To make tetra oxosulphate(VI) acid - this is the most imperative utilization of sulfur.
-
To make calcium hydrogen trioxosulphate(IV), Ca(HSO3)2 - this is utilized as a bleacher of wood pulp in the manufacture of paper.
-
In the vulcanization of rubber, that is, making the rubber tough and hard by binding the rubber molecules close to each other.
-
In dye manufacturing.
-
In the fabrication of sulfur compounds, for example, carbon disulfide, CS2 and sulfur monochloride, S2Cl2 - utilized as a part of the vulcanization of rubber since it acts as a solvent for rubber.
-
Utilized as ointments.
-
Utilized as sulfides, such as phosphorus sulfide, which is utilized as a part of making firecrackers, gunpowder and matches.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free