Sulphuric Acid
Table of Content |
About Sulphuric Acid
Sulphuric acid, H2SO4 is an odorless, colorless, oily liquid that is extremely corrosive It was at first called Oil of Vitriol.
-
It occurs in both combined and free state.
-
It is framed close to the sulfide beds.
-
In the joined frame, it happens as metallic sulfates (salts in minerals).
Fig. 1: A molecule of sulphuric acid
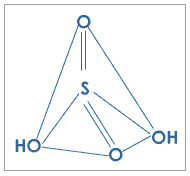
Fig. 2: Structure of sulfuric acid
Manufacture of Sulphuric Acid
Commercially, Sulphuric Acid can be prepared by two techniques that are as follows:
-
Lead chamber process
-
Contact process
Contact Process
The contact process includes three steps:
Step – I:
Production of Sulphur Dioxide:
This can be made by smoldering sulfur or sulfide ores, for example, iron pyrites in abundance of air.
S (Sulphur) + O2 (Oxygen) + Δ (Heating) → SO2 (Sulphur dioxide)
4FeS (Iron pyrites) + 7O2 (Oxygen) + Δ (heating) → 2Fe2O3 (Ferric Oxide) + 4SO2(Sulphur dioxide)
Step -II:
Formation of Sulphur Trioxide:
Sulphur dioxide is oxidized to sulphur trioxide with atmospheric oxygen within the sight of V2O5 as a catalyst.
2SO2 (Sulphur dioxide) + O2 (Oxygen) + V2O5 (Catalyst) → SO3 (Sulphurtriioxide)
Step -III
Conversion of Sulphur Trioxide into Sulphuric Acid:
The sulfur trioxide shaped in the second step is broken up in 98% sulphuric acid to give pyrosulphuric acid or oleum. Oleum is then diluted or weakened with water to give sulphuric acid of the concentration that is required.
SO3 (Sulphur trioxide) + H2SO4 (Sulphuric acid-98%) → H2S2O7 (Pyrosulphuric acid -Oleum)
H2S2O7 (Pyrosulphuric acid -Oleum) + H2O (Dilution ) → 2H2SO4(Sulphuric acid)
Fig. 3: A schematic diagram of a contact-process sulfuric acid converter
Fig. 4: Reactions and steps involved in the Contact Process
Lead Chamber Process
Lead Chamber process is one of the sulphuric acid manufacturing strategies which give just around 50-60 B grade acids. The fundamental standard of the procedure is that the wet SO2 (Sulfur Dioxide) in the nearness of nitrogenous oxides (dynamic impetus) is oxidized to sulfur trioxide with the oxygen exhibit in air. Sulfur trioxide is made to react with water to create sulphuric acid. The reactions would resemble beneath:
Fig. 5: Lead chamber process
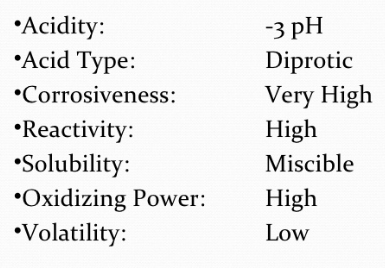
Physical Properties of Sulphuric Acid
-
Sulphuric acid is a thick, colourless, oily fluid with a specific gravity of 1.84 at 298 K.
-
The boiling point is 611 K. The high boiling point and thick nature is because of hydrogen bonding.
-
Concentrated sulphuric acid has a strong water affinity. A lot of heat is created when the acid is blended with water.
-
The acid is constantly diluted by adding it to water gradually with steady blending.
Remember - Never add water to Sulphuric Acid.
Fig. 6: Physical properties of Sulphuric Acid
Chemical Properties of Sulphuric Acid
Fig. 7: Chemical properties of Sulphuric Acid
Sulphuric Acid is a very strong dibasic acid. It ionizes in two stages in aqueous solution:
Step 1:
H2SO4(aq) + H2O(I) → H3O+(aq) + HSO4-(aq);
Ka1 = very large (Ka1 > 10)
Step 2:
HSO4(aq) + H2O(I) → H3O+(aq) + SO42-(aq);
Ka2 = 1.2 x 10-2
- The acid neutralizes alkalis and structures two arrangement of salts bisulphates and sulfates.
Example:
Concentrated sulphuric acid reacts with sodium hydroxide and structures sodium bisulphate and sodium sulfate.
H2SO4 (Conc.Sulphuric acid) + 2NaOH (Sodium hydroxide)→ 2NaHSO4 (Sodium bisulphate) + H2O (Water)
H2SO4 (Conc.Sulphuric acid) + 2NaOH (Sodium hydroxide) → Na2SO4 (Sodium sulphate) + 2H2O (Water)
- Sulphuric acid is utilized as a part of the preparation of more volatile acids from their comparing salts as it is less volatile.
- Concentrated Sulphuric acid liberates hydrogen chloride from sodium chloride and hydrogen fluoride from calcium fluoride.
CaF2 (Calcium fluoride)+ H2SO4 (Conc. Sulphuric acid) → CaSO4(Calcium sulphate) + 2HF (Hydrogen floride)
- Concentrated Sulphuric acid is an intense dehydrating agent. This property is utilized as a part of drying many wet gasses that don't react with the acid.
- Sulphuric acid additionally expels water from natural mixes like starches.
Example:
- It burns glucose, sugar, and starch to carbon.
C12H22O11(Cane sugar) + (H2SO4) → 12C (Carbon) + 11H2O (Water)
- Hot concentrated Sulphuric acid is a decent oxidizing agent. It oxidizes both nonmetals as well as metals and itself gets reduced to sulfur dioxide.
Example of Sulphuric Acid
Hot concentrated sulphuric acid oxidizes copper to copper sulfate. Similarly, it oxidizes non-metallic carbon to carbon dioxide.
Cu (Copper) + 2H2SO4 (Hot suphuric acid) → CuSO4 (Copper sulphate) + SO2 (Sulphur dioxide) + H2O (Water)
C (Carbon) + 2H2SO4 (Hot suphuric acid) → CO2 (Carbon dioxide) + 2SO2 (Sulphur dioxide) + 2H2O (Water)
- Sulphuric acid is utilized as a part of the preparation of more volatile acids from their comparing salts as it is less volatile.
Example:
Concentrated sulphuric acid liberates hydrogen chloride from sodium chloride and hydrogen fluoride from calcium fluoride.
CaF2 (Calcium fluoride)+ H2SO4 (Conc. Sulphuric acid) →
CaSO4 (Calcium sulphate) + 2HF (Hydrogen floride)
- Concentrated sulphuric acid is an intense dehydrating agent. This property is utilized as a part of drying many wet gasses that don't react with the acid.
- Sulphuric acid additionally expels water from natural mixes like starches.
Example:
- It burns glucose, sugar, and starch to carbon.
C12H22O11 (Cane sugar) + (H2SO4) → 12C (Carbon) + 11H2O (Water)
- Hot concentrated sulphuric acid is a decent oxidizing agent. It oxidizes both nonmetals as well as metals and itself gets reduced to sulfur dioxide.
Hot concentrated sulphuric acid oxidizes copper to copper sulfate .Similarly, it oxidizes non-metallic carbon to carbon dioxide.
Cu (Copper) + 2H2SO4 (Hot suphuric acid) → CuSO4 (Copper sulphate) + SO2 (Sulphur dioxide) + H2O (Water)
C (Carbon) + 2H2SO4 (Hot suphuric acid) → CO2 (Carbon dioxide) + 2SO2 (Sulphur dioxide) + 2H2O (Water)
Uses of Sulphuric Acid
Sulphuric acid, on account of its wide applications, is alluded as the King of Chemicals.
It is utilized as a part in the preparation of:
-
Fertilisers like ammonium sulfate and superphosphate
-
Dyes, shades, and paints
-
Explosives, for example, TNT
-
Other imperative chemicals like hydrochloric acid, phosphoric acid, nitric acid, and sodium carbonate
-
It is utilized as a part of the refining of petroleum
-
As a pickling agent
-
As a laboratory agent, and an oxidizing and dehydrating agent
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free