Classification of Crystalline Solids
Table of contents |
Classification of Crystalline Solids
Most of the solids found in daily life and practice are crystalline in nature. For Example metals like silver, copper, and iron, non-metals like iodine, sulphur and iodine and several compounds like NaCl (Sodium Chloride/Common Salt) etc. fall into the category of crystalline solids. We know that attraction nature of constituent particle is mainly responsible for the existence of solids. On the basis of nature of force operating between constituent particles of matter, crystalline solids are classified into four categories, namely:
Image 1: Crystalline Solids have regular arrangement of constituent particles
Molecular Solids
In molecular solids, the constituent particles of matter are molecules. Molecules are tightly packed and stacked up to form a molecular solid. Molecular Solids are further divided into three categories, on the basis of nature of molecules.
-
Non-polar Molecular Solids
-
Polar Molecular Solids
-
Hydrogen-Bonded Molecular Solids
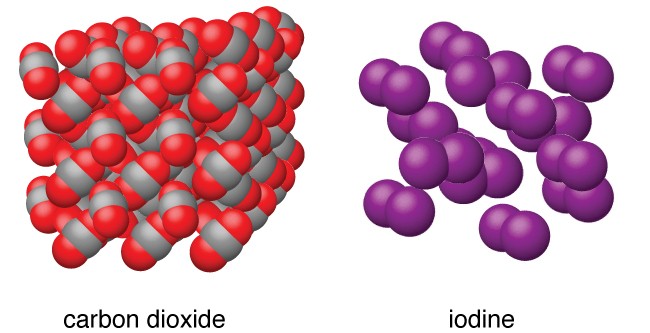
Non-polar Molecular Solids
The solids in which the molecules are made up of same kinds of atoms are called Non-molecular Solids. The dipole moment of non-polar molecular solids is zero and the forces acting between constituent molecules are weak London dispersion forces.
The characteristics of non-polar molecular solids are as follows:
-
Non-polar molecular solids are soft in nature
-
They don’t conduct electricity
-
They have low melting point and are stable in liquid and gaseous state at standard pressure and room temperature
Example: Hydrogen (H2), Chlorine (Cl2) and Iodine (I2)
Image 2: Molecular structure of CO2 and I2
Polar Molecular Solids
The solids in which the molecules are held together by strong dipole-dipole forces are known as Polar Molecular Solids. The atoms combined to form molecules are different in nature, For Example, sulphur and oxygen atoms combine to form SO2 which is a polar molecular solid.
Polar Molecular Solids depict the following properties:
-
Polar Molecular Solids are soft
-
They are non-conductors of electricity that is, they don’t conduct electricity
-
The melting point is generally higher than that of non-polar molecular solids
-
Polar Molecular Solids exist in liquid and gaseous state at standard room temperature and pressure
Example: Sulphur Dioxide (SO2) and Ammonia (NH3)
Hydrogen-Bonded Molecular Solids
In Hydrogen-bonded molecular solids the forces responsible for molecular attraction are hydrogen bonds. The boiling and melting point of hydrogen-bonded molecular solids are higher than that of polar and non-polar molecular solids.
Following characteristics are shown by Hydrogen-Bonded Molecular Solids
-
Hydrogen-Bonded Molecular Solids are non-conductor of electricity
-
They remain as soft solid and volatile liquids under specific temperature and pressure
Example: Ice (H2O)
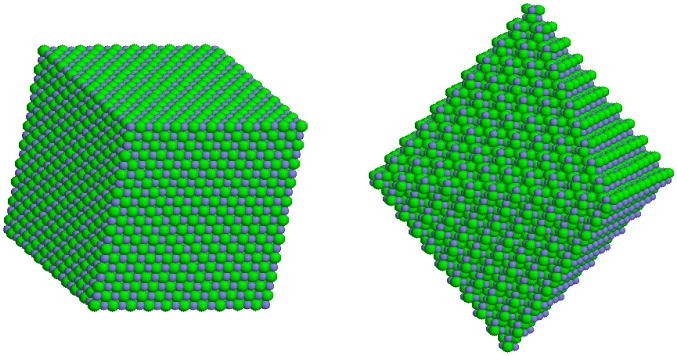
Ionic Solids
The solids in which the ions are the constituent particles are called Ionic Solids. Ionic Solids come to formation due to three-dimensional arrangement of cations and anions. The forces operating between cations and anions are electrostatic forces of attraction. These forces are quite strong in nature.
Example: Sodium Chloride (NaCl) (where Na+ is the cation and Cl- is the anion), Calcium Fluoride (CaF2) etc. fall into the category of ionic solids.
Image 3: Structure of NaCl
Ionic solids depict following properties, namely:
-
Ionic Solids are hard and brittle in nature
-
Ionic Solids have high melting points and low volatility due to high enthalpy of fusion and vaporization
-
They are poor conductors of electricity in solid state, but when we dissolve or melt them they act as good conductors of electricity ( as the ions become free to move and conduct electricity)
-
Ionic Solids are soluble in polar solvents like water
Metallic Solids
In metallic solids, the constituent particles are metal atoms and free electrons. The arrangement is made such that the metal atoms remain stationary at fixed positions and electrons are free to move inside the lattice. The bonds which are responsible for the existence of metallic solids are strong metallic bonds. Due to the presence of free electrons, metallic solids can easily conduct electricity and heat.
Example: Copper, Gold, Zinc etc.
Image 4: Electrons are free to move inside a metallic solid, thereby making it good conductor of electricity
Common properties of metallic solids are as follows:
-
Metallic Solids range from partially soft to very hard in shape
-
They are malleable (can be cut into sheets) and ductile (can be drawn into wires)
-
They are good conductors of heat and electricity due to availability of free electrons
-
They shine quite well due to their lustrous behavior
-
Metallic Solids have high melting and boiling point
-
Metallic Solids show moderate fusion enthalpies
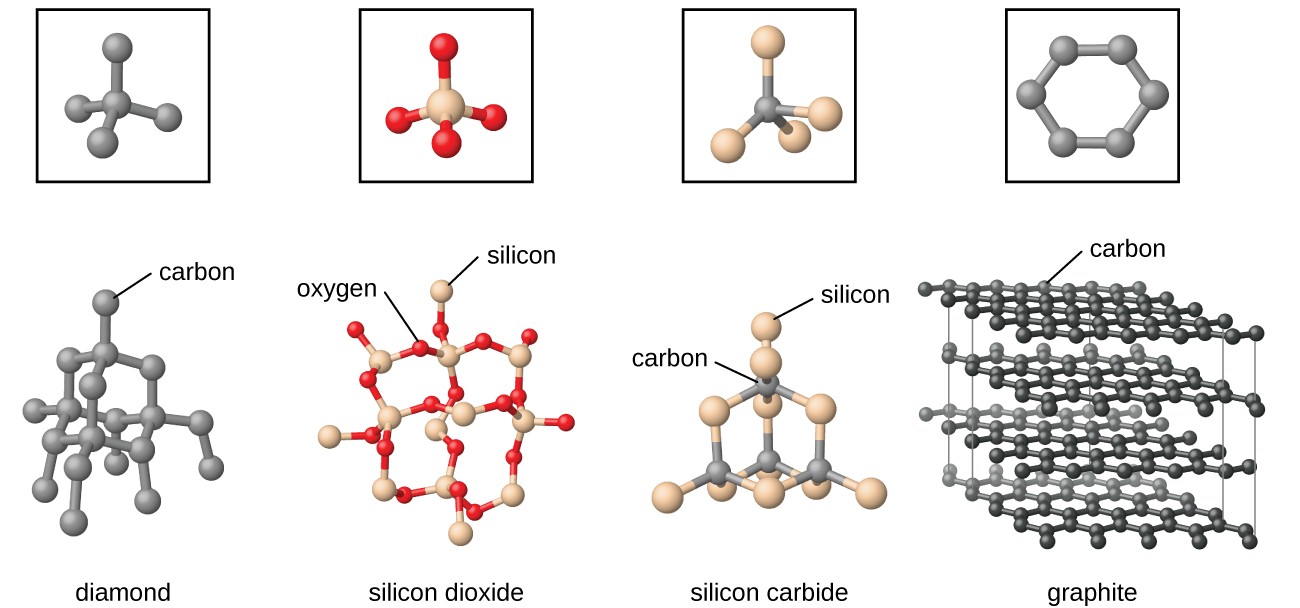
Network or Covalent Solids
In covalent solids, atoms of same and different elements are interlinked to each other to form a covalent bond network. They are also known as Giant Molecules. The interparticle forces acting between them are covalent bonds. Covalent Solids are also known as network solids because the covalent bonds form a giant interlocked network like structure.
Examples: Graphite, Diamond, Silica etc.
The properties shown by covalent solids are as follows:
-
Covalent Solids are direction and strong in nature
-
They are very hard and brittle, for example, diamond is the hardest substance in the world.
-
Covalent Solids have high melting points
-
Covalent Solids are poor conductors of heat and electricity, exception is graphite which is a good conductor of electricity
-
They show high enthalpy of fusion
Note: Although graphite is a covalent solid it shows two exceptional properties due to its typical structure, they are:
-
Graphite is soft
-
Graphite can conduct electricity
Image 5: The physical structure of graphite makes it different from other covalent solids
Why is graphite soft?
Graphite is made up of carbon atoms. The arrangement of carbon atoms is such that graphite forms a 2-Dimensional planar structure. This leads to the formation of different planar layers which can slide over each other. This makes graphite soft in nature.
Why can Graphite conduct electricity while diamond can’t?
The reason is the difference in the physical structure of graphite and diamond. In diamond the physical structure is three-dimensional in nature, that is, all the four valencies of carbon atoms are interlinked to each other. Due to close interlinked packing, there is no free electron available to conduct the electricity. Whereas in graphite, the structure is two-dimensional in nature that is, only three valencies are available for the interlinking of the covalent network and one valency is available for the conduction of electricity.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free