Imperfections in solids: Defects in Crystals
Table of Content |
 The
The  discovery of imperfections in an other wise ideally perfect crystal is one of the most fascinating aspects of solid state science. An ideally perfect crystal is one which has the same unit cell and contains the same lattice points throughout the crystal. The term imperfection or defect is generally used to describe any deviation of the ideally perfect crystal from the periodic arrangement of its constituents.
discovery of imperfections in an other wise ideally perfect crystal is one of the most fascinating aspects of solid state science. An ideally perfect crystal is one which has the same unit cell and contains the same lattice points throughout the crystal. The term imperfection or defect is generally used to describe any deviation of the ideally perfect crystal from the periodic arrangement of its constituents.
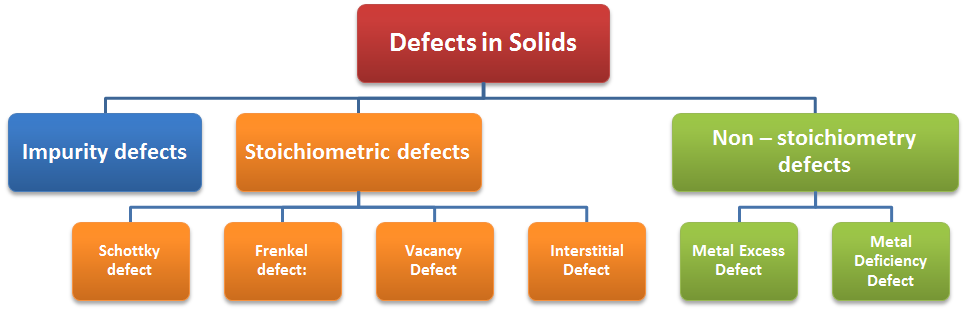
Atomic Imperfections / Point Defects
If the deviation occurs because of missing atoms, displaced atoms or extra atoms, the imperfection is named as a point defect.
Such defects can be the result of imperfect packing during the original crystallisation or they may arise from thermal vibrations of atoms at elevated temperatures because with increase in thermal energy there is increased probability of individual atoms jumping out of their positions of lowest energy.
Type of point defects – point defects in a crystal may be classified into three types
-
Stoichiometric defects
-
Non – stoichiometry defects
-
Impurity defects
Stoichiometry Defects
The compounds in which the number of cation and anions are exactly in the same ratio as represented by their chemical formula are called stoichiometric compounds. The defects that do not disturb the ratio of cations and anions are called stoichiometric defect.
-
Vacancy Defect
-
Interstitial Defect
-
Schottky Defect
-
Frenkel Defect
Vacancy Defect
-
When some of the lattice sites are vacant, the crystal is said to have vacancy defect .
-
This results in decrease in density of the substance.
 Interstitial Defect
Interstitial Defect
-
When some constituent particles (atoms or molecules) occupy an interstitial site, the crystal is said to have interstitial defect
-
This defect increases the density of the substance.
Schottky Defect
If in an ionic crystal of the type A+ B-, equal number of cations and anions are missing from their lattice. It is called Schottky defect.
This type of defect is shown by highly ionic compounds which have
-
High Co – ordination number and
-
Small difference in the sizes of cations and anions
- A few examples of ionic compounds exhibiting Schottky defect are NaCl, KCl, KBr and CsCl.
Consequences of Schottky Defect
-
As the number of ions decreases as a result of this defect, the mass decreases whereas the volume remains the same. Hence density of the solid decreases
-
The crystal begins to conduct electricity to a small extent by ionic mechanism
-
The presence of too many voids lowers lattice energy and the stability of the crystal
Watch this Video for more reference
Frenkel Defect
If an ion is missing from its correct lattice sites (causing a vacancy or a hole) and occupies an interstitial site, electrical neutrality as well as stoichiometry of the compounds are maintained.
This type of defect is called Frenkel defect. Since cations are usually smaller it is more common to find the cations occupying interstitial sites.
This type of defect is present in ionic compounds which have
-
Low co ordinations number
-
Larger difference in size of cation and anions
-
Compounds having highly polarising cation and easily polarisable anion. A few examples of ionic compounds exhibiting this defect are AgCl, AgBr, AgI, ZnS etc.
Consequences of Frenkel defect
-
As no ions are missing from the crystal lattice as a whole, therefore density of the solid remains the same
-
The closeness of like charges tends to increases the dielectric constant of the crystal
-
The crystal conducts electricity to a small extent by ionic mechanism
Non – Stoichiometric Defects
If as a result of imperfection, the ratio of number of cation to anion becomes different from that indicated by the ideal chemical formula, the defects are called non – stoichiometric defects.
These defects arise either due to excess of metal atoms or non metal atom or presence of impurities / foreign particle.
-
Metal Excess Defects. The Colour Centres
It has been observed that if a crystal of NaCl is heated in sodium vapour, it acquires a yellow colour.
This yellow colour is due to the formation of a non-stoichiometric compound of sodium chloride in which there is a slight excess of sodium ions.
What happens in this case is that some sodium metal gets doped into sodium chloride crystal which, due to the crystal energy, gets ionised into Na+ and e–. This electron occupies a site that would otherwise be filled by a chloride ion, as illustrated in figure.
There is evidently an excess of metal ions although the crystal as a whole is neutral. A little reflection would show that there are six Na+ sites adjacent to the vacant site occupied by the electron.
The extra electron in thus shared between all the six Na+ ions which implies that this electron is not localised at the vacant Cl– site. On the other hand, this electron is similar to the delocalised p electrons present in molecules containing conjugate double bonds.
Light is absorbed when this delocalised electron makes an easy transition from its ground state to an excited state. As a result, the non – stoichiometric form of sodium chloride appears coloured. Because of this, the sites occupied by the extra electrons are known as colour centres. These are also called F-centres. This name comes from the German word Farbe meaning colour.
The non-schiometric sodium chloride may be represented by the formula Na(1+d)Cl where d is the excess sodium metal doped in the crystal because of its exposure to sodium vapour.
Another common example of metal excess defects is the formation of a magenta coloured non-stoichiometric compound of potassium chloride by exposing the crystals of KCl to K metal vapour.
The coloured compound contains an excess of K+ ions, the vacant Cl– sites being filled by electrons obtained by the ionization of the excess K metal doped in to the crystal.
-
Metal Deficiency Defects
 In certains cases, one of the positive ions is missing from its lattice site and the extra negative charge is balanced by some nearby metal ion acquiring two charges instead of one.
In certains cases, one of the positive ions is missing from its lattice site and the extra negative charge is balanced by some nearby metal ion acquiring two charges instead of one.
There is evidently, a deficiency of the metal ions although the crystal as a whole is neutral. This type of defect is generally found amongst the compounds of transition metals which can exhibit variable valency.
Crystals of FeO, FeS and NiO show this type of defects. The existence of metal deficiency defects in the crystal of FeO is illustrated.
It is evident from the above discussion that all types of point defects result in the creation of vacancies or ‘holes’ in the lattice of the crystals.
The presence of holes lowers the density as well as the lattice energy or the stability of the crystals. The presence of too many holes may cause a partial collapse of the lattice.
Solved Example |
|
Question: Titanium monoxide has a rock-salt structure. X-ray diffraction data show that the length of one edge of the cubic unit cell for TiO with a 1:1 ratio of Ti to O is 4.18Å, and the density as determined by volume and mass measurements is 4.92 g cm–3. Do the data indicate that defects are present? If so, are they vacancy or interstitial defects? [Ti = 47.88 u]. Solution: The presence of vacancies (Schottky defects) at the Ti and O sites should be reflected in a lower measured density than that calculated from the size of the unit cell and the assumption that every Ti and O site is occupied. Interstitial (Frenkel) defects would give little of any difference between the measured and theoretical densities. There are four formula units per unit all so theoretical density is d = 4 (47.88+16) / 6.023 × 1023 (4.18 × 1023) = 4.81g cm–3 This is significantly greater than the measured density. The crystal must, therefore, contain numerous vacancies. Because the overall composition of the solid is TiO, there must be equal number of vacancies on cation and anion sites. |

Question 1: Which of the following defects is not a type of Stoichiometry defects ?
a. Vacancy defect
b. Interstitial Defect
c. Schottky defect and
d. Metal excess defect
Question 2: In which of the following defects equal number of anions and cations are missing from lattice site?
a. Vacancy defect
b. Interstitial defect
c. Schottky defect and
d. Frenkel Defect
Question 3: Which of the following defects does not cause any change in density of crystal??
a. Vacancy defect
b. Interstitial defect
c. Schottky defect and
d. Frenkel Defect
Question 4: The metal excess defect, the sites occupied by the extra electrons are known as
a. f- centres
b. vacant site.
c. interstitial site
d. site of defect
Question 5: Which of the following compounds does not show metal deficiency defect?
a. FeO
b. FeS
c. NiO

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
Q.5 |
|
d |
c |
d |
a |
d |
Related Resource
-
Look here for Syllabus of IIT JEE
-
Click here to get the Past year Papers of IIT JEE
-
Know about Useful Books of Chemistry
To read more, Buy study materials of Solid State comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More