Number of Atoms in a Unit Cell
Table of contents |
Introduction to Number of Atoms in a Unit Cell
We know that a crystal lattice comprises of several unit cells. In a unit cell, every constituent particle( atom, molecule or ion ) has a specific and fixed position called lattice site. We can calculate a number of atoms/molecules and ions in a unit cell easily by analyzing the nature and position of constituent particles in unit cells.
Image 1: Types of Unit Cell
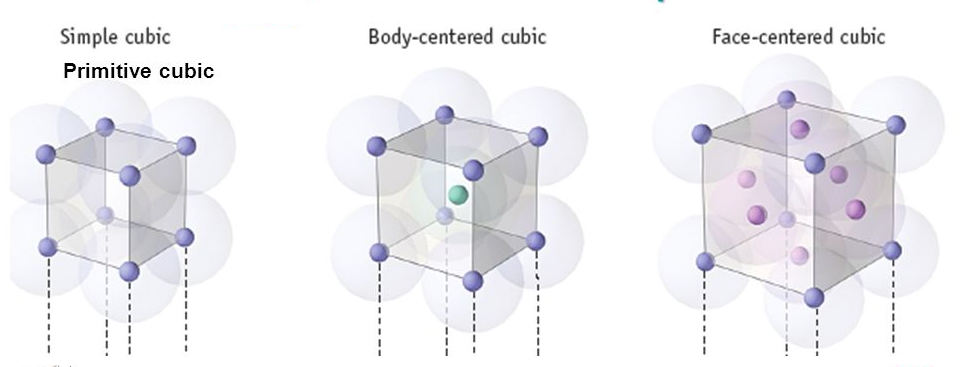
Primitive Cubic Unit Cell
In the primitive cubic unit cell, the atoms are only located on the corners. That means 8 atoms are located on 8 corners of the lattice. Each atom located on the corner contributes 1/8th of the original volume of the cell. So since there are total 8 atoms in a primitive cubic unit cell, the total number of atoms in the primitive cubic unit cell.

So there is only 1 atom in a primitive cubic unit cell.
Image 2: The corners get only 1/8th part of atom
Body- centred Cubic Unit Cell
In a body-centred unit cell, 8 atoms are located on the 8 corners and 1 atom is present at the center of the structure. So total atoms in the body-centred unit cell will be:
Since 8 atoms are present at the corners, each will contribute 1/8th of the original volume of the cell. Thus in the body-centred cubic unit cell:
- There are 8 corners and 1 corner shares 1/8th volume of the entire cell, so

-
Also, the atom at the centre is wholly present at the centre of the cell and can’t be shared
1 × 1 = 1 atom
So there are total 2 atoms present in a body centred unit cell.
Image 3: Atoms in body-centred unit cell
Face-centred Cubic Unit Cell
In face-centred cubic unit cell atoms are present on 8 corners and center of all the faces. Also, each atom located on the centre of the unit cell is shared by two adjacent unit cells. Therefore only half atom belongs to a single unit cell.
Thus in Face-centred cubic unit cell
-
There are 8 atoms present on 8 corners, therefore, each corner will get 1/8 part of atom

-
There are six faces and each face gets 1/2 part of atom then

Total atoms present in a face-centred unit cell = 1 + 3 = 4 atoms
End-centred Cubic Unit Cell
In end-centred cubic unit cell, 8 atoms are located on 8 corners of the cube and 1 atom each is present on two opposite faces of the cube.
Therefore in end-centred cubic unit cell
-
There are 8 atoms present on 8 corners, therefore each atom contributes 1/8th portion of the cell

-
There are 2 atoms located at the center of the cell and each atom contributes 1/2 portion of the cell

Total atoms present in a end-centred cubic unit cell = 1 + 1 = 2 atoms
Image 4: Contribution of Atom in different unit cells
The table given below summarizes a total number of atoms present in a unit cell.
|
Type of Unit Cell |
Number of atoms at corners |
Number of atoms on faces |
Number of atoms in center |
Total |
|
Simple Cubic |
8 × 1/8 = 1 |
0 |
0 |
1 |
|
Body Centred Cubic |
8 × 1/8 = 1 |
0 |
1 |
2 |
|
Face Centred Cubic |
8 × 1/8 = 1 |
6 × 1/2 = 3 |
0 |
4 |
|
End Centred Cubic |
8 × 1/8 = 1 |
2 × 1/2 = 1 |
0 |
2 |
Image 5: Types of Unit Cell
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free