Packing Fractions
Table of Content |
-
Both hcp & ccp though different in form are equally efficient. They occupy the maximum possible space which is about 74% of the available volume. Hence they are called closest packing.
-
In addition to the above two types of arrangements a third type of arrangement found in metals is body centred cubic (bcc) in which space occupied is about 68%.
-
Packing Efficiency =

Let us calculate the packing efficiency in different types of structures
Simple Cubic Unit Cell
 Let ‘a’ be the edge length of the unit cell and r be the radius of sphere.
Let ‘a’ be the edge length of the unit cell and r be the radius of sphere.
As sphere are touching each other
Therefore a = 2r
No. of spheres per unit cell = 1/8 × 8 = 1
Volume of the sphere = 4/3 πr3
Volume of the cube = a3= (2r)3 = 8r3
∴ Fraction of the space occupied = 1/3πr3 / 8r3 = 0.524
∴ % occupied = 52.4 %
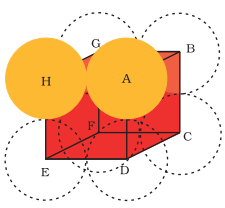
Body-centred Cubic Unit Cell
 In body centred cubic unit cell
In body centred cubic unit cell
In 
Let DF= b
and we know that
ED=EF= a (edge length)
Now,
b2 = a2 + a2 = 2a2
In 
Let, AF = c
We know that
FD = b
& AD = a (edge length)
Now,
c2 = a2 + b2 = a2 + 2a2 = 2a2
or c = √3 a
we know that c is body diagonal. As the sphere at the centre touches the sphere at the corner. Therefore body diagonal c = 4r
i.e. √3 a = 4r
or r = (√3/4)a
or a = 4r / √3
∴ Volume of the unit cell = a3 = (4r / √3)3 = 64r3 / 3√3
No. of spheres in bcc = 2
∴ volume of 2 spheres = 2 × 4/3πr3

Face-Centred Cubic (hcp and ccp Structures)
 Let ‘r’ be the radius of sphere and ‘a’ be the edge length of the cube
Let ‘r’ be the radius of sphere and ‘a’ be the edge length of the cube
As there are 4 sphere in fcc unit cell
∴ Volume of four spheres = 4 (4/3 πr3)
In fcc, the corner spheres are in touch with the face centred sphere. Therefore, face diagonal AD is equal to four times the radius of sphere
AC= 4r
AC = √AD2 + DC2 = √a2 + a2= √2a
4r = √2a
or a = 4/√2 r
∴ volume of cube = (2/√2 r)3

Watch this Video for more reference
| Thus, it is concluded that ccp and hcp structures have maximum packing efficiency. |
Calculation Involving Unit Cell Dimensions
From the unit cell dimensions, it is possible to calculate the volume of the unit cell. Knowing the density of the metal. We can calculate the mass of the atoms in the unit cell. The determination of the mass of a single atom gives an accurate determination of Avogadro constant.
Suppose edge of unit cell of a cubic crystal determined by X – Ray diffraction is a, d is density of the solid substance and M is the molar mass, then in case of cubic crystal
Volume of a unit cell = a3
Mass of the unit cell = no. of atoms in the unit cell × mass of each atom = Z × m
Here Z = no. of atoms present in one unit cell
m = mass of a single atom
Mass of an atom present in the unit cell = m/NA

| Density of the unit cell is same as the density of the substance. |

Question 1: Packing efficiency of simple cubic unit cell is ..
a. 74.05%
b. 64.54%
c. 78.00%
d. 52.40%
Question 2: Which of the following crystal systems has minimum packing efficiency?
a. HCP
b. CCP
c. BCC
d. Simple Cubic
Question 3: Which of the following cubic unit cell has packing efficiency of 64%?
a. HCP
b. CCP
c. BCC
d. Simple Cubic
Question 4: For BCC unit cell edge length (a) =
a. 4r
b. 4r / √3
c. 4/√2 r
d. 2r
Question 5: For FCC unit cell, volume of cube =
a. 64 / 2√2 r3
b. 64r3 / 3√3
c. 25r3 / 3√3

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
Q.5 |
|
d |
d |
c |
b |
a |
Related Resources
-
You can also refer to Syllabus of chemistry for IIT JEE
- Click here to get the Past year Papers of IIT JEE
-
Look here for Crystal Lattices and Unit Cells
To read more, Buy study materials of Solid State comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free