Properties of Solids
Table of Content |
 The three main properties of solids which depend upon their structure
The three main properties of solids which depend upon their structure
-
Electrical properties
-
Magnetic properties
-
Dielectric properties
Electrical Properties of Solids
-
Electrical conductivity of solids may arise through the motion of electrons and positive holes (electronic conductivity) or through the motions of ions (ionic conductivity).
-
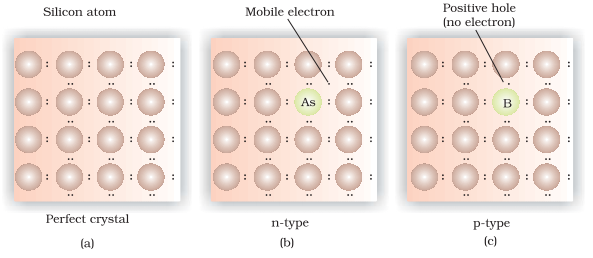
The conduction through electrons is called n-type conduction and through positive holes is called p – types conduction.
-
Electrical conductivity of metal is due to motion of electrons and it increases with the number of electrons available to participate in the conduction process.
-
Pure ionic solids where conduction can take place only through motion of ions are insulators. However, the presence of defects in the crystal structure increases their conductivity.
On the basis of electrical conductivity the solids can be classified into three types
-
Metal (conductors): They allow the maximum portion of the applied electric field to flow through them and have conductivities in order of 106 – 108 ohm-1.
-
Insulators: They have low conductivities i.e. they do not practically allow the electric circuit to flow through them. The electrical conductivity is in order 10-10 – 10-20 ohm-1 m-1
-
Semi conductors: The solids with intermediate conductivities at the room temperature. Semi conductors allow a portion of electric current to flow through them.
Actually semi conductors are those solids which are perfect insulators at absolute zero, but conduct electric current at room temperature.
-
Intrinsic semi conductors (semi-conductors due to thermal defects)
At zero Kelvin pure substance silicon and germanium act as insulators because electrons fixed in covalent bonds are not available for conduction. However at higher temperature some of the covalent bonds are broken and the electrons so released become free to move in the crystal and thus conduct electric current. This type of conduction is known as intrinsic conduction as it can be introduced in the crystal without adding an external substance.
-
Extrinsic semi conductors: (semi conductors due to impurity defects)
The conductivity of pure silicon and germanium is very low at room temperature. The conductivity of silicon and germanium can be increased by doping with impurities producing n-type semiconductors or p – type semi conductors
Watch this Video for more reference
Magnetic Properties of Solids
-
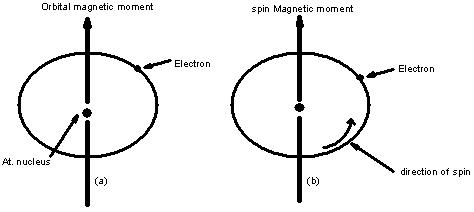
 The magnetic properties of different materials are studies in terms of their magnetic moments which arise due to the orbital motion and spinning motion of the electron.
The magnetic properties of different materials are studies in terms of their magnetic moments which arise due to the orbital motion and spinning motion of the electron. -
As electron is charged particle, the circular motion of the electric charge causes the electron to act as a tiny electro magnet.
-
The magnetic moment of the magnetic field generated due to orbital motion of the electron is along the axis of rotation.
-
The electron also possesses magnetic moment due to the spin which is directed along the spin axis.
-
Thus, magnetic moment of the electron is due to travelling in closed path (orbital motion) about the nucleus and spinning on its axis.
-
For each electron spin magnetic moment is ±μB Where μB, Bohr Magneton is the fundamental unit of magnetic moment and is equal to 9.27 × 10-24 em2.
-
The magnetic moment due to orbital motion is equal to Mlμ B where Ml is the magnetic quantum number of the electron.
-
As magnetic moment is a vector quantity, the net magnetic moment of an electron may be represented by an arrow.
-
Thus a material may be considered to contain a number of magnetic dipoles (similar to a bar magnet with north and south poles).
-
Due to the magnetic moment of the electrons different substances behave differently towards the external applied magnetic field.
Based on the behaviour in the external magnetic field, the substances are divided into different categories as explained below.
- Diamagnetic substance:
Substances which are weakly repelled by the external magnetic fields are called diamagnetic field e.g. TiO2, NaCl, benzene etc. Diamagnetic substances have all their electrons paired.
- Paramagnetic substances:
Substances which are weakly attracted by magnetic field are called paramagnetic substances. These substance have permanent magnetic dipoles due to the presence of some species (atoms, ions or molecules) with unpaired electron. The paramagnetic substances lose their magnetism in the absence of magnetic field. For e.g. TiO, VO2 and CuO, O2, Cu+2, Fe+3 etc.
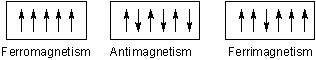
- Ferromagnetic substances:
Substances which show permanent magnetism even in the absence of the magnetic field are called Ferromagnetic substances. e.g. Fe Ni. CO, CrO2 show Ferromagnetism. Such substances remain permanently magnetised, once they have been magnetised. This type of magnetism arises due to spontaneous alignment of magnetic moment due to unpaired electrons in the same direction.
- Anti Ferromagnetic substance
Substances which are expected to possess paramagnetism or Ferromagnetism on the basis of unpaired electron but actually they posses zero net magnetic moment are called anti Ferromagnetic substances e.g. MnO, Mn2O3, MnO2.
Anit Ferromagnetism is due to presence of equal number of magnetic moments in the opposite direction.
- Ferrimagnetic substances
Substance which are expected to posses large magnetism on the basis of the unpaired electrons but actually have small net magnetic moments are called Ferrimagnetic substances e.g. Fe3O3
Dielectric Properties
A dielectric substance is, in which an electric field gives rise to no net flow of electric charge. This is due to the reason that electrons in a dielectric substances are tightly held by individual atoms. However when electric field is applied. Polarization takes place because nuclei are attracted to one side and the electron cloud to the other side. In addition to these dipoles, there may also be permanent dipoles in the crystal.
The alignment of these dipoles may be in compensatory way i.e. the net dipole moment is zero or noncompensatory way i.e. has a net dipole moment. The net dipole moment leads to certain characteristic properties to solids.
- Piezoelectricity (or pressure electricity)
When mechanical stress is applied on crystals so as to deform them, electricity is produced due to displacement of ions. The electricity thus produced is called piezoelectricity and the crystals are called piezoelectric crystals. Conversely, if electric field is applied to such crystals, atomic displacement takes place resulting into mechanical strain. This is sometimes called Inverse piezoelectric effect.
The crystals are used as pick – ups in record players where they produce electrical signals by application of pressure. Examples of piezoelectric crystals include titanates of barium and lead, lead zirconate (PbZrO3), ammonium dihydrogen phosphate (NH4H2PO4) and quartz. They are also used in microphones, ultrasonic generators and sonar detectors.
- Pyroelectricity:
Some piezoelectric crystals when heated produce a small electric current. The electricity thus produced is called pyroelectricity.
- Ferroelectricity:
In some of the piezoelectric crystals, the dipoles are permanently polarized even in the absence of the electric field. However on applying electric field, the direction of polarization changes e.g. Barium titanate (BaTiO3) sodium potassium tartarate (Rochelle salt) and potassium dihydrogen phosphate (KH2PO4). All ferroelectric solids are piezoelectric but the reverse is not true.
- Anti Ferroelectricity:
In some crystals, the dipoles align themselves in such a way, that alternately, they point up and down so that the crystal does not posses any net dipole moment. Such crystal are said to be anti Ferroelectric e.g. Lead zirconate (PbZrO3)
Super Conductivity
-
 A substance is said to be superconducting when it offers no resistance to the flow of electricity.
A substance is said to be superconducting when it offers no resistance to the flow of electricity. -
Electrical resistance decreases with decreases in temperature and becomes almost zero near the absolute zero.
-
The phenomenon was first discovered by Kammerlingh Onnesin 1913 when he found that mercury becomes superconducting at 4 K.
-
The temperature at which a substance starts behaving as super conductor is called transition temperature.
-
Most metals have transition temperatures between 2K -5K. Certain organic compounds also becomes superconducting below 5K. Such low temperature can be attained only with liquid helium which is very expensive.
-
Super conductivity materials have great technical potentials. They can be used in electronics in building magnets, in power transmission and levitation transportation (trains which move in air without rails).
|
|
Solved Example |
Question.Explain: (a) The basis of similarities and difference between metallic and ionic crystals. (b) Unit cell is not simply a cube of 4Na+ ions and 4Cl- ions. (c) Can a cube consisting of Na+ and Cl- ions at alternate corners serve as satisfactory unit cell for the sodium chloride lattice? (d) Ionic solids are hard and brittle. Solution:(a) Similarities:
Differences: Ionic bond is a strong bond due to electrostatic forces of attraction while metallic bond may be weak or strong depending upon the kernels. (b) Unit cell of NaCl has fcc arrangement of Cl- ions and Na+ ions are present at the edge centres and one at the body-cnetre. Thus there are 14Cl- ions and 13Na+ ions in the unit cell. However their net contribution towards the unit cell is 4Na+ and 4Cl- ions. (c) Yes because its repetition in different directions produces the complete space lattice. (d) Ionic solids are hard because there are strong electrostatic forces of attraction. However they are brittle because the bond is non-directional. |

Question 1: Conductors have conductivities in order of
a. 106 – 108 ohm-1
b. 100 – 108 ohm-1
c. 10 – 108 ohm-1
d. 10 – 1018 ohm-1
Question 2: Bohr Magneton is the fundamental unit of magnetic moment and is equal to
a. 1.27 × 10-24 em2t
b.9.27 × 10-24 em2
c. 9.1 × 10-24 em2
d. 2.1 × 10-24 em2
Question 3: Some piezoelectric crystals when heated produce a small electric current. The electricity thus produced is called .
a. piezoelectricity
b. pyroelectricity
c. ferroelectricity
d. pyroelectricity
Question 4: Which of the following substances is not paramagnetic?
a. TiO,
b. VO2
c. CrO2
d. CuO
Question 5: TiO2, is
a. diamagnetic
b. paramagnetic
c. ferromagnetic

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
Q.5 |
|
a |
b |
d |
c |
a |
Related Resources
-
Look here for Syllabus of IIT JEE
-
Click here to get the Past year Papers of IIT JEE
-
Know about Structure of Ionic Compounds
To read more, Buy study materials of Solid State comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More