Solubility
Table of Content |
 What is Solubility?
What is Solubility?
The maximum amount of a solute that can be dissolved in each volume of solution at a constant temperature and pressure is called Solubility.
Let’s do a Case Study based on Solubility…
During Summer Vacation, one day you and your best friend were doing some school assignment, since it was too warm both took out a chilled coke can from refrigerant. As the can lid was opened a brisk fizz was heard followed by bubbling in  it. You both takes a sip and it was refreshing. Suddenly you heard a voice from outside and you both were needed there without delay. In hurry, you put your opened can on the same place where you were working, however your friend chooses to put back the open can in refrigerator before moving out.
it. You both takes a sip and it was refreshing. Suddenly you heard a voice from outside and you both were needed there without delay. In hurry, you put your opened can on the same place where you were working, however your friend chooses to put back the open can in refrigerator before moving out.
Indeed, it was a clear move by your friend to put the can back in refrigerator, do you know why?
After an hour when you both came back inside you both just grabbed your drink respectively. Firstly, when you took a long gulp from your can. Not only it was warm, but it was flat too lacking fizz. It was because all the fizz that’s the trapped Carbon dioxide gas has escaped out.
Secondly when your best friend takes his previously used can out of refrigerator, and he drinks some. Not only it was cold but was amazingly fizzy even. So how can we explain this difference? Why in latter case carbon dioxide is still there, any idea? Let’s see below:
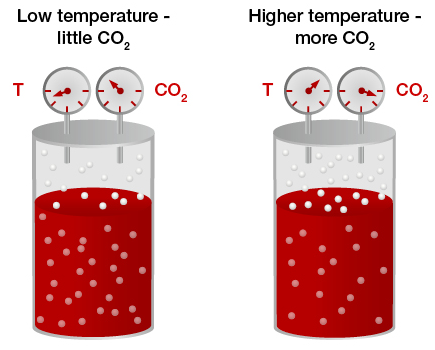
It was due to solubility of gas in liquid, solubility is the interaction between solvent and the particles of the solute. Hence it depends on the nature of both the solute and solvent, as well as on the pressure and temperature. In the above case as temperature increases the solubility decreases.
 |
 |
|
Fig: Solubility of gases in solvent. |
Types of Solubility:
1. Solubility of Solid Solute in Liquid Solvent:
Factors affecting solubility of solid solute in liquid solvent:
-
Nature of the solute and solvent.
-
Effect of Temperature.
-
Nature of the Solute and Solvent

There is a scientific proverb that “LIKE DISSOLVE LIKE*” Keeping this as bench mark, we can tell that ionic/polar compounds like NaCl, KCl gets dissolved in polar solvent like water and the non-polar/covalent or organic molecules can dissolve in non-polar compounds like benzene can be dissolved in acetone.
LIKE DISSOLVE LIKE Principle-IT DEPENDS ON TYPES OF BOND / polarity of molecules / IMF BETWEEN SOLUTES AND SOLVENT.
- Nature of solute
The solutes (solids) can be classified as ionic and non-ionic solids. The ionic solids consist of positively and negatively charged ions. It is the force of attraction between the ions, i.e., lattice energy which opposes the tendency of a solute to dissolve. This force of attraction is different in different ionic solids depending on the charges present on the ions and distance between ions (ionic radii). The ionic solutes having high less lattice energy have more solubility. The ions are solvated by the solvent molecules and in this process energy (known as hydration energy) is released. When the hydration energy is high, the ionic solid is more soluble. Many non-ionic substances dissolve in polar solvents due to hydrogen bonding. Generally, if the solute and solvent have similar characteristics, i.e. both polar or both non-polar, the solubility is high and if both are dissimilar, the solubility is found low.
- Nature of solvent
Ionic solids dissolve to a larger extent in a solvent having a high dielectric constant as compared to solvents of low dielectric constants. Dielectric constant of water is 80 while that of methyl alcohol is 33.5 an ionic solid, therefore, dissolves more readily in water than in methyl alcohol. Benzene has a very low dielectric constant of 2.3 and, hence, ionic solids do not dissolve in benzene. For non-ionic solids, the guiding principle is ‘like dissolves like, i.e., if the solvent is polar, it will dissolve the polar solutes and if it is non-molar, it will dissolve the non-polar solutes in it.
-
Effect of temperature: It has 3 cases.
- The solubility of solid is directly proportional to temperature when the process of dissolution is endothermic (When heat is absorbed).
(Solute + Solvent + Heat → Solution)
For Example: NaCl, KCl etc.
- The solubility of solid decreases with increase of temperature when the process of dissolution is exothermic (When heat comes out).
| (Solute + Solvent → Heat + Solution) |
For Example: Na2CO3, H2O etc
- Solids whose solubility does not increase or decrease with temperature fluctuation.
For Example: CaCl2, 6H2O
Figure denotes How Solid Solutes get dissolved in Liquid Solvents
Solubility of solid in liquid is also dependent on:
-
Nature of Solute-Amorphous or crystalline
-
Surface area of solute
-
Amount of solvent used
-
Container size
2. Solubility of Gas in Liquid
Factors affecting solubility of gaseous solute in liquid solvent:
i. Temperature
Cold Liquid can dissolve more amount of gas but as temperature rises, the solubility of gas decreases. Rise in temperature causes an elevation in kinetic energy of gas molecules due to fast motion of gas molecules. Increased kinetic energy causes fast motion and the molecules collide with each other and even with wall of container this break intermolecular bonds and the gases escapes out easily from solution.
Examples we see around:
-
Recall the cold drink can scenario when you open and keep it at room temperature the dissolved CO2 escapes and the drink gives a very flat taste and non-fizzy.
-
Thermal power plants that discharges the hot water into water bodies like ponds / rivers / lakes may kill the fish by decreasing the dissolved oxygen concentration in it.
-
Imagine how the fishes and other aquatic life survive inside the water bodies in polar areas where water if freezed on top.
ii. Pressure:
Gases like hydrogen, oxygen, nitrogen etc. get mixed/dissolved in water to a small extent but gases like NH3, CO2, HCl are highly soluble in water.
The higher solubility of these gases CO2, NH3, HCl are because they react with solvent. This greater solubility of the gas in a solvent is due to their chemical similarity.
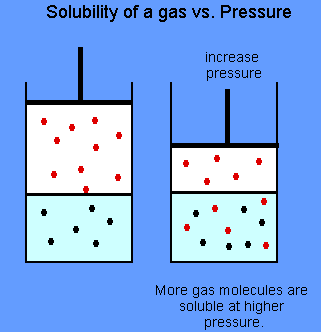
It has been found that the gas solubility in liquid increases with increase in pressure
The effects of pressure on the solubility of gases in liquid can be best described through a combination of Henrys law and Le Châtelier principle.
It was given by William Henry in 1803 and it states that:
“The amount of gas that dissolves in a given liquid is directly proportional to the partial pressure of that gas.”
OR
“The mass of a gas dissolved by a given volume of a liquid, at constant temperature, is proportional to the pressure of the gas”.
It is used to do solubility based calculations
Mathematical form of Henrys law / Henrys Solubility Formula:
| P = KHC |
P = Partial pressure of the solute.
C = Is the concentration of the solute.
KH = Henrys law constant which is different for each solute-solvent pair.
It has been observed that most gases obey Henrys law provided,
-
The pressure is not too high.
-
The temperature is not too low.
Applications of Henrys Law
-
To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
-
At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Example of Solubility
All carbonated drinks are bottled under high pressure to increase the CO2 solubility in solution. When the bottle is opened, the pressure above the solution decreases which leads in the solution effervescence / fizz and some of carbon dioxide bubbles off.
Which medical situation is seen to many scuba divers or what is “Bend’s Disease”?
Scuba divers may experience a health condition called “bends” if they fail to readjust quite slowly to the lower pressure at the surface when he comes up. As a result of inhaling compressed air and subjected to high pressure caused by water depth, the amount of nitrogen present in blood and other tissues increases. If the diver come back to the surface rapidly, the mixed nitrogen gas in blood forms bubbles and it becomes less soluble due to lowering in pressure. The nitrogen bubbles can cause great pain and possible death.
To solve this problem, breathing mixture of oxygen and helium are used. Helium is only 1/5th as soluble in blood as nitrogen. Thus, there is less dissolved gas to form bubbles.
Solutions of Liquids in Liquids
When one liquid dissolves in another, the molecules of the solvent are caused to move apart so as to accommodate the solute molecules. Similarly, the solute molecules must also be separated so that they can take their places in the mixture. In both these processes energy is required. Finally, as the solute and solvent molecules are brought together, energy is released because of the attractive forces between them. When solute and solvent molecules are strongly attracted to each other, more energy is released in the final step. Three cases may arise under these circumstances. The overall dissolution process results either in evolution of heat or absorption of heat, or energy released in the final step is the same as the absorbed in the first two, i.e., net change is zero.
Examples:
-
Benzene and carbon tetrachloride: No evolution or absorption of Heat.
-
Acetone and water: Evolution of heat.
-
Ethyl alcohol and water: Absorption of heat.
A liquid may or may not be soluble in another liquid. Depending upon the relative solubility of a liquid in another, the following three cases are possible.
-
Liquids that is completely miscible. For Example: Benzene and toluene; Ethyl alcohol and water; carbon tetrachloride and benzene.
-
Liquids that is partially miscible. For Example: Ether and water; Phenol and water; Nicotine and water.
-
Liquids that is practically immiscible. For Example: Benzene and water; carbon tetrachloride and water; Benzene and alcohol.
Frquently Asked Questions (FAQs)
Q1. Which of the following factors does not affect the solubility of solids in liquid to large extent?
a. Nature of solvent
b. Temperature
c. Nature of solute
d. Pressure
Sol. d. Pressure
Q2. A solution which remains in contact with undissolved solute is termed as
a. ideal solution
b. non-ideal solution
c. saturated solution
d. unsaturated solution
Sol. c. saturated solutionc
Q3. Which of the following liquid pairs is completely miscible in each other?
a. Benzene and water
b. Carbon tetrachloride and water
c. Benzene and alcohol
d. Ethyl alcohol and water
Sol. d. Ethyl alcohol and water
Q4. To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under ..
a. high pressure and low temperature
b. high pressure and high temperature
c. low pressure and high temperature
d. low pressure and low temperature.
Sol. a. high pressure and low temperature
Watch this Video for more reference
Related Resources
-
Click here to go through syllabus of IIT JEE
-
Look here for reference books of chemistry
-
You can also refer methods of expressing concentration of solution
To read more, Buy study materials of Solutions comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More