Colligative Properties

A dilute solution is one in which the amount of the solute is very small in comparison to the amount of the solvent.The dilute solutions show more or less ideal behavior as the heat and volume changes, accompanying the mixing of solute and solvent, are negligible for all practical purposes. Dilute solutions obey Raoult’s law.
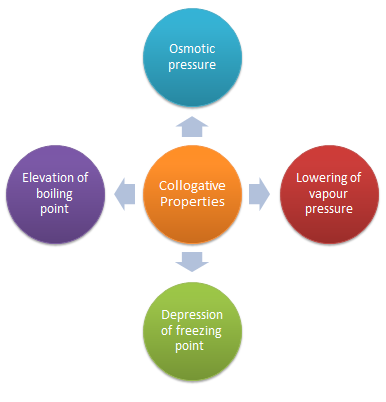
The properties of dilute solutions which depend only on number particles of solute present in the solution and not on their identity are called colligative properties (denoting depending upon collection).
We shall assume here that the solute is non volatile, so it does not contribute to the vapour. We shall also assume that the solute does not dissolve in solid solvent, that is, the pure solid solvent separates when the solution is frozen, the latter assumption is quite drastic, although it is true for many mixtures, it can be avoided.
Colligative properties are the properties of only dilute solution which are supposed to behave as ideal solutions. The various colligative properties are:
Depression of freezing point
-
Lowering of vapour pressure
-
Osmotic pressure
-
Elevation of boiling point
Lowering of Vapour Pressure by Non Volatile Solute
It has been known for a long time that when a non-volatile solute is dissolved in a liquid, the vapour pressure of the solution becomes lower than the vapour pressure of the pure solvent. In 1886, the French chemist, Francois Raoult, after a series of experiments on a number of solvents including water, benzene and ether, succeeded in establishing a relationship between the lowering of vapour pressure of a solution and the mole fraction of the non-volatile solute.
Let us consider a solution obtained by dissolving n moles of a non-volatile solute in N moles of a volatile solvent. Then mole fraction of the solvent, X 1 = N/(n+N) and mole fraction of the solute, X2 = n /(N +n). Since the solute is non-volatile, it would have negligible vapour pressure. The vapour pressure of the solution is, therefore merely the vapour pressure of the solvent. According to Raoult's law, the vapour pressure of a solvent (P1) in an ideal solution is given by the expression ;
P1 = X1 P10 ................(1)
where P10 is the vapour pressure of the pure solvent. Since X1 + X 2 = 1, Eq. 1 may be written as

The expression on the left hand side of Equation (3) is usually called the relative lowering of vapour pressure. Equation (3) may thus be stated as: `The relative lowering of vapour pressure of a solution containing a non-volatile solute is equal to the mole fraction of the solute present in the solution.'
This is one of the statements of the Raoult's law.
Since mole fraction of the solute, X 2 is given by n/(N+n), Equation (3) may be expressed as

It is evident from Equation (4) that the lowering of vapour pressure of a solution depends upon the number of moles (and hence on the number of molecules) of the solute and not upon the nature of the solute dissolved in a given amount of the solvent. Hence, lowering of vapour pressure is a colligative property.
Refer to the following video for colligative properties
Determination of Molar Masses from Lowering of Vapour Pressure
It is possible to calculate molar masses of non-volatile non-electrolytic solutes by measuring vapour pressures of their dilute solutions.
Suppose, a given mass, w gram, of a solute of molar mass m, dissolved in
W gram of solvent of molar mass M, lowers the vapour pressure from P10 to P1.
Then , by Equation (4)

Since in dilute solutions, n is very small as compared to N, Equation (5) may be put in the approximate form as

Limitations of Raoult’s Law
-
Raoult’s law is applicable only to very dilute solutions.
-
It is applicable to solutions containing non-volatile solute only.
-
It is not applicable to solutes which dissociate or associate in a particular solution.
Example 1 : |
|
Question The density of a 0.438 M solution of potassium chromate at 298 K is 1.063 g cm-3. Calculate the vapour pressure of water above this solution. Given : P0 (water) = 23.79 mm Hg. Solution A solution of 0.438 M means 0.438 mol of K2CrO4 is present in 1L of the solution. Now, Mass of K2CrO4 dissolved per litre of the solution = 0.438 Mass of 1L of solution = 1000 Amount of water in 1L of solution = 978.028/18 = 54.255 mol Assuming K2CrO4 to be completely dissociated in the solution, we will have; Mole fraction of water solution = 54.335/(54.335+1.314) = 0.976 Finally, Vapour pressure of water above solution = 0.976 |
Boiling Point Elevation by a Non-Volatile Solute
The boiling point of a liquid is the temperature at which its vapour pressure becomes equal to 760 mm (i.e. 1 atmospheric pressure). Since the addition of a non-volatile solute lowers the vapour pressure of the solvent, the vapour pressure of a solution is always lower than that of the pure solvent, and hence it must be heated to a higher temperature to make its vapour pressure equal to atmospheric pressure. Thus the solution boils at a higher temperature than the pure solvent. If Tb0 is the boiling point of the solvent and Tb is the boiling point of the solution, the difference in boiling points ( Tb) is called the elevation of boiling point.
Tb) is called the elevation of boiling point.
 Thus, Tb –Tb0 =
Thus, Tb –Tb0 =  Tb.
Tb.
 Tb a molality where
Tb a molality where  Tb = elevation of boiling point
Tb = elevation of boiling point
n= no. of moles of non-volatile solute
N= Total no. of moles in the solution
This implies that the boiling point elevation in a dilute solution is directly proportional to the number of moles of the solute dissolved in a given amount of the solvent and is quite independent of the nature of the solute. Hence, boiling point elevation is a colligative property
 Tb = Kb
Tb = Kb  m
m
kb : molal elevation constant or Ebullioscopic constant
m : molality of the solution
Molal boiling point elevation constant or ebullioscopic constant of the solvent, is defined as the elevation in boiling point which may theoretically be produced by dissolving one mole of any solute in 1000 g of the solvent.
or

where m1 = molecular weight of solute and w and W are weights of solute and solvent.
Depression of Freezing Point by a Non-Volatile Solute
Freezing point is the temperature at which solid and liquid states of a substance have the same vapour pressure. It is observed that the freezing point of the solution (Tf) containing non volatile solute is always less than the  freezing point of the pure solvent (Tf0). Thus, Tf0 - Tf =
freezing point of the pure solvent (Tf0). Thus, Tf0 - Tf =  Tf
Tf
It can be seen that
 Tf
Tf  molality
molality
that, is freezing point depression of a dilute solution is directly proportional to the number of moles of the solute dissolved in a given amount of the solvent and is independent of the nature of solute
or  Tf = Kfm
Tf = Kfm
kf : molal freezing point depression constant of the solvent or cryoscopic constant
m : molality of the solution
Molal freezing point depression constant of the solvent or cryoscopic constant, is defined as the depression in freezing point which may theoretically be produced by dissolving 1 mole of any solute in 1000g of the solvent.

where m1 = molecular weight of solute and w and W are weights of solute and solvent.

Osmosis and Osmotic Pressure
The phenomenon of the passage of pure solvent from a region of lower concentration (of the solution) to a region of its higher concentration through a semi-permeable membrane is called osmosis.
The driving force of osmosis is what is known as osmotic pressure. It is the difference in the pressure between the solution and the solvent system or it is the excess pressure which must be applied to a solution in order to prevent flow of solvent into the solution through the semi-permeable membrane. Once osmosis is complete the pressure exerted by the solution and the solvent on the semi-permeable membrane is same.
Van't Hoff equation for dilute solutions is ( parallel to ideal gas equation)
 V = nRT
V = nRT
where 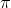 = Osmotic pressure
= Osmotic pressure
V= volume of solution
n= no. of moles of solute that is dissolved
R = Gas constant
T= Absolute temperature
Isotonic Solutions : A pair of solutions having same osomotic pressure is called isotonic solutions.
Reverse Osmosis
When a solution is separated from pure water by a semipermeable membrane, water moves towards solution on account of osmosis. This process continues till osmotic pressure becomes equal to hydrostatic pressure or osmosis can be stopped by applying external pressure equal to osmotic pressure on solution. If external pressure greater than osmotic pressure is applied, the flow of solvent molecules can be made to proceed from solution towards pure solvent, i.e., in reverse direction of the ordinary osmosis. This type of osmosis is termed reverse osmosis. Reverse osmosis is used for the desalination of sea water for getting fresh drinking water.


Determination of osmotic pressure: Berkeley and Hartley’s method
Various methods are employed for the measurement of osmotic pressure but the best known method was suggested by Berkeley and Hartley. The apparatus used is shown in Fig. 5.11. A porcelain tube having copper ferrocyanide membrane in its walls in enclosed in a metallic jacket. The porcelain tube is fitted with a reservoir of pure solvent at one end and a capillary tube at the other end. In a metallic jacket is an arrangement for applying external pressure which is measured with the help of pressure eggs.
Procedure:
The porcelain tube is filled with pure solvent and the metallic jacket with solution. The level in the capillary tube will tend to move down as the solvent flows towards solution due to osmosis. External pressure is now applied on the solution by the piston so that level in capillary remain stationary. The reading of pressure gauge is recorded. This is the osmotic pressure of the solution.

Advantages:
-
It is a quick and accurate method.
-
The concentration of the solution does not change because flow of solvent is not permitted into solution; so the results obtained by this method are reliable.
-
As the osmotic pressure is balanced by external pressure, there is no strain on membrane and the danger of its bursting is eliminated. So, this method can be used to measure high osmotic pressure also.
Comparison of osmotic pressures:
de-Vries developed an approximate method for comparing the relative osmotic pressures of aqueous solutions. A solution of low osmotic pressure is termed hypotonic and solution of high osmotic pressure is termed hypotonic. The protoplasmic layer, lining the cell walls of plant cells is easily penetrated by water but us almost impermeable to the substances dissolved in the cellular fluid.
When a plant cell is placed in hypotonic solution, the water is drawn in and the cell swells. If the cell is placed in a hypertonic solution, water will diffuse out of the cell fluid and partial collapse of the cell will take place when potonic solution is separated from hypertonic solution using semipermeable membrane then osmosis takes place from hypotonic to hypertonic because solvent concentration is greater in hypotonic solution. this phenomenon is known as plasmolysis.
The change in cell can be placed in the solution of same osmotic pressure as that of the cell fluid, no change in the structure of the cell is observed. Such solutions having some osmotic pressures are termed isotonic. By putting, therefore, cells of the same kind into solutions of different concentrations, it can be ascertained whether the solution is hypertonic or hypotonic or isotonic.

Relation between osmotic pressure and vapour pressure
Let an aqueous dilute solution filled in a capillary tube is closed at one end by semipermeable membrane. The tube is placed in pure solvent (water). Entire apparatus is closed by a belljar. At osmotic equilibrium, the belljar is saturated with water vapour. At equilibrium osmotic pressure (π) becomes equal to hydrostatic pressure.

π = h × d …(i)
where ‘h’ is height in the column, π is osmotic pressure
Let p0 = Vapour pressure of pure solvent
p = Vapour pressure of solution
Pressure at level L1 = p0; Pressure at level L2 = p. Pressure at L1 will be greater than pressure at L2. Then
P0 – p = hD …(ii)
where ‘d’ is density of solvent vapour of pressure p0.
Dividing Eq. (i) by (ii), we have,

at a fixed temperature d/D is constant.
∴ π = constant (p0 − p) …(iii)
i.e., π ∝ (p0 – p) or ![]() p …(iv)
p …(iv)
Thus, osmotic pressure is directly proportional to the lowering of vapour pressure.
We know that p0V = nRT

i.e.

 ….............(v)
….............(v)
Dividing Eq. (iii) by (v), we get


At a fixed temperature, π and M/dRT are constant.
Thus,![]() p/p0 ∝ π, i.e., Osmotic pressure is proportional to relative lowering of vapour pressure.
p/p0 ∝ π, i.e., Osmotic pressure is proportional to relative lowering of vapour pressure.

Question 1: Colligative properties depend only on
a. volume
b. pressure
c. temperature
d. number of particles
Question 2: Which of the following alternatives does not represent a colligative property?
a. Lowering of vapour pressure
b. Osmosis
c. Elevation of boiling point
d. Depression of freezing point
Question 3: The driving force of osmosis is
a. osmotic pressure
b. vapour pressure
c. surface tension
d. viscosity
Question 4: Such solutions having some osmotic pressures are termed
a. isobaric
b. isochoric
c. isotopic
d. isotonic

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
d |
b |
a |
d |
Related Resources
-
You can also refer to the syllabus of IIT JEE
-
Click here for reference books of chemistry
-
Look here for methods of expressing concentration of solution
To read more, Buy study materials of Solutions comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free
