Types of Solutions
Table of Content |
We know that a binary solution is a homogeneous mixture of two substances: a solute and a solvent
Solution components are:
Solute: is the substance being dissolved; present in less quantity.
Solvent: is the substance doing the dissolving; present in large quantity.
What are the basis of solution’s differentiation? OR
Why is Solution of so many types?
The different types are given below:
CASE – 01: On the basis of Water as Solvent
Aqueous solution: Solutions that contain water as the solvent.
Ex. sugar in water, carbon dioxide in water, etc.
Non-Aqueous Solution: Solutions that contain a solvent other than water like Ether, benzene, petrol, carbon tetrachloride etc
Ex. sulfur in carbon disulphide, naphthalene in benzene, etc.
CASE – 02: On the basis of amount of Solute Added
Unsaturated Solution: A solution is said to be unsaturated when the solvent (e.g. water) is capable of dissolving more solute (e.g. Sugar) at a (definite/fixed) temperature.
Ex: 1% NaCl solution.(1 g of NaCl in 99 ml pure water)
Saturated Solution: A solution is said to be saturated when the solvent is not capable of dissolving any more solute at a (definite/fixed) temperature.
Ex: 40% NaCl solution
Supersaturated Solution: A solution is said to be super saturated when the solute is present in excess amount and dissolved forcefully by increasing temperature or pressure. They generally crystal out in bottom by the method called crystallization.
CASE – 03: On the basis of Type/State of Solvent used
(Solutes and solvents may be of any form of matter: solid, liquid or gas)
CASE - 04: On the basis of amount of Solvent Added
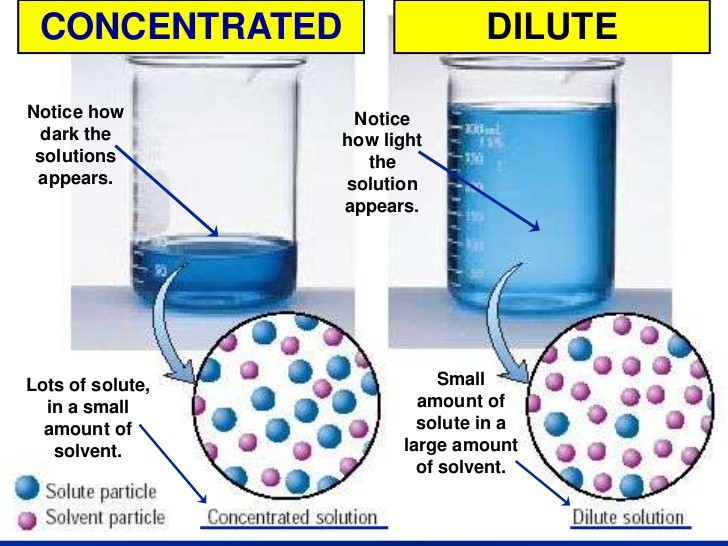
Concentrated Solution: A solution is said to be concentrated when it has large amount of solute in given solvent.
Ex: Brine solution, Orange juice, dark color tea.
Dilute Solution: A solution is said to be dilute when it contains small amount of solute in large amount of solvent.
Ex: Salt solution, light color tea
CASE-05: On the basis of Concentration of Solute in two Solutions
(Let’s consider a system containing a solution in beaker and inside that solution a Biological cell is kept, this cell contain a liquid in it, all together we have two liquids in this system)
Isotonic Solution: The two solutions that have the same concentration of a solute in it so water moves across the cell membrane in both directions maintaining cell size.
Ex: A solution of 0.89% NaCl.
Hypertonic Solution: The solution kept in beaker has higher concentration of solute in it so water comes out of the cell and into the solution in beaker causing the cell to plasmolyze/shrink
Ex: Salt solution, Corn syrup
Hypotonic Solution: The solution kept in beaker has lower concentration of solute in it so water moves into the cell causing cells to swell up and finally burst.
Ex: Water
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free