Van der Waals Forces
Table of Content |
-
 Intermolecular forces are the forces of attraction and repulsion acting between interacting particles (atoms and molecules).
Intermolecular forces are the forces of attraction and repulsion acting between interacting particles (atoms and molecules). -
This term does not include the electrostatic forces that exist between the two oppositely charged ions and the forces that hold atoms of a molecule together i.e., covalent bonds.
-
The attractive intermolecular forces are called vander Waals’ force. in honor of Dutch scientist Johannes van der Waals (1837-1923) as he explained the deviation of real gases from the ideal behaviour through these forces.
-
Van-der Waals’ s forces vary considerably in magnitude and include , dispersion forces or London forces, dipole-dipole forces, and dipole-induced dipole forces.

Instantaneous Dipole – Induced Dipole Interactions
-
The electrons of neutral molecules keep on oscillating w.r.t. the nuclei of atoms. As a result, at a given instant, one side of the molecule may have a slight excess of electrons relative to the opposite side.
-
Thus a non – polar molecule may become momentarily self – polarized.
-
This polarized molecule may induce a dipole moment in the neighboring molecule. These two induced dipoles then attract each other .
-
These momentary dipole – induced dipole attractions are also called London forces or dispersive forces.
 The magnitude of these forces depends upon the following:
The magnitude of these forces depends upon the following:
-
Size or Molecular Mass: The melting points and boiling points of non – polar molecules increase as the size or molecular mass of the molecule increases. For example, the m.p. and b.p. of alkanes, halogens, noble gases etc. increase as the molecular mass of the molecule increases.
-
Geometry / Shape : For example, isomer n – pentane has higher boiling point than neo – pentane because the former is zig – zag chain with larger sites of contact and hence large intermolecular forces whereas the latter is nearly spherical and hence has less contact and weaker forces.
Dipole – Dipole Interactions
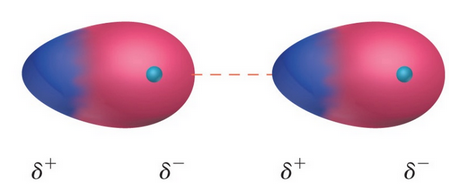
In case of polar molecules, the vander waals’ forces are mainly due to electrical interaction between oppositively charged ends of molecules called dipole – dipole interactions.
-
For example, gases such as etc.have permanent dipole moments as a result of which there is appreciable dipole – dipole interactions between the molecules of these gases.
-
The magnitude of this type of interaction depends upon the dipole moment of the molecule concerned.
-
Evidently, greater the dipole moment, stronger is the dipole – dipole interactions. Because of these attractive forces, these gases can be easily liquefied.
Dipole – Induced Dipole Interactions
-
A polar molecule may sometimes polarize a non – polar molecule which lies in its vicinity and thus induces polarity in that molecule just as a magnet induces magnetic polarity in a neutral piece of iron lying close by.
-
The induced dipole then interacts with the dipole moment of the first molecule and thereby the two molecules are attracted together.
-
The magnitude of this interaction, evidently depends upon the magnitude of the dipole moment of the polar molecule and the polarizability of the non – polar molecule.
-
The solubility of inert gases in increases from He to Rn due to a corresponding increase in magnitude of the dipole – induced dipole interactions as the polarizability of the noble gas increases with increase in size from He to Rn.
Watch this Video for more reference
Examples |
|
Question 1: Why diethyl ether has higher vapour pressure than ethyl alcohol at a particular temperature. Solution: This is because the intermolecular forces of attraction in ethyl alcohol are stronger than those present in diethyl ether. ___________________________ Question 2: Why falling liquid drops are spherical? Solution: This is due to property of surface tension possessed by the liquids. This makes the surface area minimum. For a given volume, sphere has minimum surface area. ___________________________ Question 3: Arrange the following in the increasing order of melting point of different types of crystalline solid (i) Covalent solid (ii) Metallic solid (iii) Molecular (iv) Ionic. Solution: Covalent > Ionic > Metallic > Molecular (4000 K) (1000 K) (880 – 1000 K) (273 – 600 K) _______________________________________ Question 4: Explain (a) Aerated water bottles are kept under water during summer. (b) A bottle of liquid ammonia is cooled before opening the seal. Solution: (a) Aerated water contains dissolved in water. The solubility of gas decreases with rise in temperature. Thus, amount of free gas in bottle can increase in summer, which may result into increase in pressure and ultimately explosion. To avoid this they are kept under cold water. (b) If the seal is opened without cooling, the liquid ammonia suddenly vaporises and gushes out of the bottle with force. This may lead to serious accidents |

Question 1: Which of the following molecules will show dipole – dipole Interactions ?
a. H2
b. O2
c. CH4
d. HCl
Question 2: Which of the following interactions is also known as London forces?
a. Dipole-dipole interactions
b. Ion- dipole interactions
c. Dipole – Induced Dipole Interactions.
d. Instantaneous Dipole – Induced Dipole Interactions:
Question 2: Which of the following interactions is not a Vander wall force?
a. Dipole-dipole interactions
b. Electrostatic Interactions
c. Dipole – Induced Dipole Interactions.
d. Instantaneous Dipole – Induced Dipole Interactions:
Question 4: Which of the following type of interactions may exist between water and xenone molecule?
a. Dipole-dipole interactions
b. Ion- dipole interactions
c. Dipole – Induced Dipole Interactions.
d. Instantaneous Dipole – Induced Dipole Interactions

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
d |
d |
b |
c |
Related Resources
-
Click here to go through Syllabus for IIT JEE,
-
Look here for Reference books of Chemistry
-
You can also refer Kinetic Molecular Theory
To read more, Buy study materials of States of Matter comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More