Energy Levels of Hydrogen Atom
Table of Content |
In accordance to Bohr’s atomis model,
 (a) The central part of the atom called nucleus, contains whole of positive charge and almost whole of the mass of atom. Electrons revolve round the nucleus in fixed circular orbits.
(a) The central part of the atom called nucleus, contains whole of positive charge and almost whole of the mass of atom. Electrons revolve round the nucleus in fixed circular orbits.
(b) Electrons are capable of revolving only in certain fixed orbits, called stationary orbits or permitted orbits. In such orbits they do not radiate any energy.
(c) While revolving in permitted orbit an electron possesses angular momentum ‘L’ (=mvr) which is an integral multiple of h/2π.
(d) Electrons are capable of changing the orbits. On absorbing energy they move to a higher orbit while emission of energy takes place when electrons move to a lower orbit.
(e) All the laws of mechanics can be applied to electgron revolving in a stable orbit while they are not applicable to an electron in transition.
Hydrogen atom has atomic number ‘Z’ as one. It contains one electron revolving round the nucleus. The spectrum of H-atom studied by Lyman, Balmer, Paschen, Bracken and Pfund can now be explained on the basis of Bohr's Model. It is now clear that when an electron jumps from a higher energy state to a lower energy state, the radiation is emitted in form of photons. The radiation emitted in such a transition corresponds to the spectral line in the atomic spectra of H-atom.
As the transition of electron takes place from a higher orbit to a lower orbit, difference of energy is radiated in the form of radiation. The wavelength of the radiation depends upon the initial and final orbit within which the transition takes place. Accordoingly a number of series are emitted. Each series is composed of a umber of lines.
Watch this Video for more reference
Lyman Series
When an electron jumps from any of the higher states to the ground state or 1st state (n = 1), the series of spectral lines emitted lies in ultra-violet region and are called as Lyman Series. The
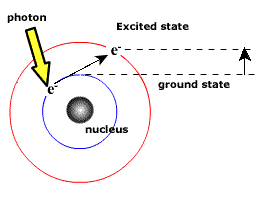 wavelength (or wave number) of any line of the series can be given by using the relation:
wavelength (or wave number) of any line of the series can be given by using the relation:
 = RZ2 (1/12 – 1/n22), n2 = 2, 3, 4, 5, ...
= RZ2 (1/12 – 1/n22), n2 = 2, 3, 4, 5, ...
(For H atom Z = 1)
Series limit (for H - atom):  –> 1 i.e.
–> 1 i.e. = R
= R
α line: 2 —> 1; also known as first line or first member
β line: 3 —> 2; also known as second line or second member
γ line: 4 —> 1; also known as third line or third member
Balmer Series
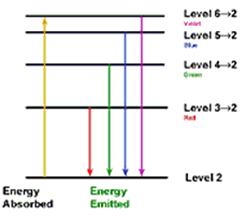 When an electron jumps from any of the higher states to the state with n = 2 (IInd state), the series of spectral lines emitted lies in visible region and are called as Balmer Series. The wave number of any spectral line can be given by using the relation:
When an electron jumps from any of the higher states to the state with n = 2 (IInd state), the series of spectral lines emitted lies in visible region and are called as Balmer Series. The wave number of any spectral line can be given by using the relation:
 = RZ2 (1/22 – 1/n22), n2 = 3, 4, 5, 6, ...
= RZ2 (1/22 – 1/n22), n2 = 3, 4, 5, 6, ...
Series limit (for H – atom) :  –> 2 i.e.
–> 2 i.e.  = R/4
= R/4
α line : 3 –> 2; β line : 4 –> 2; γ line : 5 –> 2
Paschen Series
When an electron jumps from any of the higher states to the state with n = 3 (IIIrd state), the series of spectral lines emitted lies in near infra-red region and are called as Paschen Series.  The wave number of any spectral line can be given by using the relation:
The wave number of any spectral line can be given by using the relation:
 = RZ2 (1/32 – 1/n22), n2 = 4, 5, 6, 7, ...
= RZ2 (1/32 – 1/n22), n2 = 4, 5, 6, 7, ...
Series limit (for H – atom) :  –> 3 i.e.
–> 3 i.e.  = R/9
= R/9
α line : 4 –> 3; β line : 5 –> 3; γ line : 6 –> 3
Brackett Series
When an electron jumps from any of the higher states to the state with n = 4 (IVth state), the series of spectral lines emitted lies in far infra-red region and called as Brackett Series. The wave number of any spectral line can be given by using the relation:
 = R Z2 (1/42 – 1/n22), n2 = 5, 6, 7, 8, …
= R Z2 (1/42 – 1/n22), n2 = 5, 6, 7, 8, …
P-fund Series
When an electron jumps from any of the higher states to the state with n = 5 (Vth state), the series of spectral lines emitted lies in far infra-red region and are called as P-fund Series. The wave number of any spectral line can be given by using the relation:
 = R Z2 (1/52 – 1/n22), n2 = 6, 7, 8, …
= R Z2 (1/52 – 1/n22), n2 = 6, 7, 8, …
Problem (JEE Advanced):
In hydrogen and hydrogen like atoms the ratio of difference of energies E4n- E2n nad E2n-En varies with atomic number Z and principal quantum number n as,
(a) Z2/n2 (b) Z4/n4
(c) Z/n (d) None of these
Solution:
For hydrogen atom, En= E1/n2,
So, E4n- E2n/E2n-En
= [(E1/16n2) – (E1/4n2)]/[(E1/4n2) – (E1/n2)]
= ¼ (Which is a constant)
Thus from the above observation we conclude that, option (d) is correct.
|
|


Question 1
The central part of the atom is called,
(a) electron (b) nucleus
(c) proton (d) neutron
Question 2
Balmer series lies in
(a) visible region (b) infrared region
(c) ultraviolet region (d) none of the above
Question 3
P fund series lies in
(a) ultraviolet region (b) visible region
(c) infrared region (d) none of the above
Question 4
Paschen series lies in
(a) visible region (b) infrared region
(c) ultraviolet region (d) none of the above


| Q.1 | Q.2 | Q.3 | Q.4 |
|
b |
a |
c |
b |
Related Resources
-
You might like to refer Nucleus.
-
For getting an idea of the type of questions asked, refer the Previous Year Question Papers.
-
Click here to refer the most Useful Books of Physics.
- To get answer to any question related to energy levels of hydrogen atom click here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More


