Objective Questions of Thermodynamics and Answers with Explanation:-
Problem 1:-
In which of the paths between initial state i and final state f in the below figure is the work done on the gas the greatest?
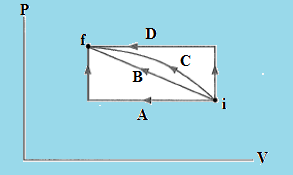 Solution:-
Solution:-
The correct option is (D).
Work is a path function. So work done on the gas depends upon the path. The area under pv diagram gives the work done on the gas between initial state i and final state f. As the area under the path D is greater than the other path, therefore the paths between initial state i and final state f where the work done on the gas is greatest would be D. From the above observation we conclude that, option D is correct.
_____________________________________________________________________
Problem 2:-
A 1-kg block of ice at 0ºC is placed into a perfectly insulated, sealed container that has 2 kg of water also at 0ºC. The water and ice completely fill the container, but the container is flexible. After some time one can except that
(A) the water will freeze so that the mass of the ice will increase.
(B) the ice will melt so that the mass of the ice will decrease.
(C) both the amount of water and the amount of ice will remain constant.
(D) both the amount of water and the amount of ice will decrease.
Solution:-
The correct option is (B) the ice will melt so that the mass of the ice will decrease.
In accordance to second law of thermodynamics, if an irreversible process occurs in a closed system, the entropy of that system always increases; it never decreases. Entropy measures the state of disorder. As the ice is more order than water, therefore the ice will melt so that the mass of the ice will decrease. From the above observation we conclude that, option (B) is correct.
________________________________________________________________________________
Problem 3:-
Which of the following processes must violate the first law of thermodynamics? (There may be more than one answer!)
(A) W > 0, Q < 0, and ?Eint = 0
(B) W > 0, Q < 0, and ?Eint > 0
(C) W > 0, Q < 0, and ?Eint < 0
(D) W < 0, Q > 0, and ?Eint < 0
(E) W > 0, Q > 0, and ?Eint < 0
Solution:-
The correct option is (E).
In accordance to first law of thermodynamics, for a thermodynamic system, in which internal energy is the only type of energy the system may have, the law of conservation of energy may be expressed as
Q + W = ΔEint
Here Q is the energy transferred (as heat) between the system and environment, W is the work done on (or by) the system and ΔEint is the change in the internal energy of the system.
By convention we have chosen Q to be positive when heat is transferred into the system and W to be positive when work is done on the system.
In the option (E), W > 0 and Q > 0. If we put this condition in the left hand side of the equation Q + W = ΔEint, then the right hand side of the equation must be greater than zero (ΔEint > 0). Thus W > 0, Q > 0, and ΔEint < 0 must violate the first law of thermodynamics. Therefore option (E) is correct.
________________________________________________________________________________
Problem 4:-
A spherical constant temperature heat source of radius r1 is at the center of a uniform solid sphere of radius r2. The rate at which heat is transferred through the surface of the sphere is propertional to
(A) r22 – r12
(B) r2 – r1
(C) ln r1 – ln r2
(D) 1/r2 – 1/r1
(E) (1/r2 – 1/r1)-1
Solution:-
The correct option is (A).
The rate H at which heat is transferred through the slab is,
(a) directly proportional to the area (A) available.
(b) inversely proportional to the thickness of the slab Δx.
(c) directly proportional to the temperature difference ΔT.
So, H = kA ΔT/ Δx
Where k is the proportionality constant and is called thermal conductivity of the material.
From above we know that, the rate H at which heat is transferred through the slab is directly proportional to the area (A) available.
Area A of solid sphere is defined as,
A = 4πr2
Here r is the radius of sphere.
So, the area A1 of uniform small solid sphere having radius r1 will be,
A1 = 4πr12
And, the area A2 of uniform large solid sphere having radius r2 will be,
A2 = 4πr22
Thus the area A from which heat is transferred through the surface of the sphere will be the difference of area of uniform large solid sphere A2 and small solid sphere A1.
So, A = A2 - A1 = 4πr22 - 4πr12 = 4π (r22 - r12)
Since the rate H at which heat is transferred through the slab is directly proportional to the area (A) available, therefore the rate at which heat is transferred through the surface of the sphere is proportional to r22 - r12.
From the above observation we conclude that, option (A) is correct.
____________________________________________________________________________________
Problem 5:-
What would be the most likely value for CT, the molar heat capacity at constant temperature?
(A) 0
(B) 0 < CT < Cv
(C) Cv < CT < Cp
(D) CT = 
Solution:-
The correct option is (A).
Molar heat capacity at constant temperature (CT) is defined as the amount of heat required to raise the temperature of 1 g of gas through 1° C keeping its temperature constant. But it is impossible to rise the temperature of 1 g of gas through 1° C keeping its temperature constant. So the two statements contradict to each other. Thus the most likely value for molar heat capacity at constant temperature (CT) will be zero. Therefore option (A) is correct.
_______________________________________________________________________________________---
Problem 6:-
Which type of ideal gas will have the largest value for Cp – Cv?
(A) Monoatomic
(B) Diatomic
(C) Polyatomic
(D) The value will be the same for all.
Solution:-
The correct option is (D).
The specific heat at constant volume (Cv) for monoatomic gas is,
Cv = 3/2 R
The specific heat at constant volume (Cv) for diatomic gas is,
Cv = 5/2 R
The specific heat at constant volume (Cv) for polyatomic gas is,
Cv = 3 R
But we know that, for an ideal gas,
Cp - Cv = R
So,the specific heat at constant pressure (Cp) for monoatomic gas will be,
Cp = Cv + R = 3/2 R + R = 5/2 R
the specific heat at constant pressure (Cp) for diatomic gas will be,
Cp = Cv + R = 5/2 R + R = 7/2 R
the specific heat at constant pressure (Cp) for polyatomic gas will be,
Cp = Cv + R = 3 R + R = 4 R
Therefore,
Cp - Cv for monoatomic gas will be,
Cp - Cv = 5/2 R - 3/2 R = R
Cp - Cv fo diatomic gas will be,
Cp - Cv = 7/2 R - 5/2 R = R
Cp - Cv fo polyatomic gas will be,
Cp - Cv = 4 R - 3 R = R
From the above observation we conclude that, the value of Cp - Cv will be same for all ideal gas. Therefore option (D) is correct.
__________________________________________________________________________________________________
Problem 7:-
Consider the following processes that can be done on an ideal gas: constant volume, ΔV = 0; constant pressure, Δp = 0; and constant temperature, ΔT = 0.
(a) For which process does W = 0?
(b) For which process does Q = 0?
(c) For which of these process does W + Q = 0?
(d) For which of these process does ΔEint = Q?
(e) For which of these process does ΔEint = W?
(A) ΔV = 0 (B) Δp = 0 (C) ΔT = 0 (D) None of these
Solution:-
(a) The correct option is (A) ΔV = 0.
The change in work done ΔW is defined as,
ΔW = pΔV
Therefore W = 0 for the process where ΔV = 0. From the above observation we conclude that, option (A) is correct.
In adiabatic process the exchange of heat with surrounding is zero (Q=0). In this process, the change of volume, pressure and temperature occurs. Therefore option (D) is correct.
For a thermodynamic system, in which internal energy is the only type of energy the system may have, the law of conservation of energy may be expressed as,
Q + W = ΔEint
Here Q is the energy transferred (as heat) between the system and its environment, W is the work done on or by the system and ΔEint is the change in the internal energy of the system.
As W + Q = 0, it signifies that ΔEint = 0 and it is possible only when ΔT = 0. Therefor option (C) is correct.
For a thermodynamic system, in which internal energy is the only type of energy the system may have, the law of conservation of energy may be expressed as,
Q + W = ΔEint
Here Q is the energy transferred (as heat) between the system and its environment, W is the work done on or by the system and ΔEint is the change in the internal energy of the system.
As ΔEint = Q, it signifies that W=0.
The change in work done ΔW is defined as,
ΔW = pΔV
Therefore W = 0 for the process where ΔV = 0. From the above observation we conclude that, option (A) is correct.
For a thermodynamic system, in which internal energy is the only type of energy the system may have, the law of conservation of energy may be expressed as,
Q + W = ΔEint
Here Q is the energy transferred (as heat) between the system and its environment, W is the work done on or by the system and ΔEint is the change in the internal energy of the system.
As ΔEint = W, it signifies that Q=0.
In adiabatic process the exchange of heat with surrounding is zero (Q=0). In this process, the change of volume, pressure and temperature occurs. Therefore option (D) is correct.
_________________________________________________________________________________________
Problem 8:-
Consider an ideal heat pump and a perfect electric heater. The electric heater converts 100% of the electrical energy into heat energy; the heat pump converts 100% of the electrical energy into work, which then powers a Carnot refrigerator. Which is the more “efficient” way to heat a home? (Ignore maintenance or start-up costs.)
(A) The electric heater is always more efficient.
(B) The heat pump is always more efficient.
(C) The heat pump is more efficient if the outside temperature is not too cold.
(D) The heat pump is more efficient if the outside temperature is not too cold.
Solution:-
The correct option is (C).
A heat pump is a device that acting as a Carnot refrigerator can heat a house by transferring heat energy from the outside to the inside of the house; the process is driven by work done on the device. So, heat pump, which is also a refrigerator, is an air conditioner that can be operated in reverse to heat room. By heating the room using a perfect electric heater, there is some loss of thermal energy due to heating the coil of the electric heater.
The value of coefficient of performance K becomes larger as the temperature of the two reservoirs becomes more nearly the same. Thus heat pumps are more efficient in temperate climates than in climates where the outside temperature fluctuates between wide limits. From the above observation we conclude that, the heat pump is more efficient if the outside temperature is not too warm. Thus option (C) is correct.
______________________________________________________________________________________________
Problem 9:-
A real engine operates at 75% of the efficiency of a Carnot engine operating betwen the same two temperatures. This engine has a power output of 100 W and discharges heat into the 27ºC low-temperature reservior at a rate of 300 J/s. what is the temperature of the high-temperature reservior?
(A) 27ºC (B) 77ºC (C) 127ºC (D) 177ºC
Solution:-
The correct option is (C) 127 ° C.
The efficiency ε of a Carnot engine is defined as,
ε = |QH| -|QL|/|QH|
=1- |QL|/|QH|
= 1- TL/TH
Here QL is the heat at lower temperature, QH is the heat at higher temperature, TL is the lower temperature and TH is the higher temperature.
As the real engine has a power output of 100 W (100 J/s) and discharge heat into the 27 ° C low-temperature reservoir at a rate of 300 J/s.
Here, |QH| -|QL| = 100 J
And
|QL| = 300 J
So, |QH| -300 J = 100 J
|QH| = 400 J
To obtain the efficiency ε of this real engine, substitute 300 J for QL and 400 J for QH in the equation ε = 1- |QL/QH|,
ε = 1- |QL/QH|
= 1-300 J/400 J
= 1/4
To obtain the temperature of the high-temperature region TH, substitute 1/4 for ε, 27 ° C for TL in the equation ε =1- TL/TH we get,
1/4=1- TL/TH
= 1-27 ° C/ TH
= 1- (27+273) K/ TH
= 1- 300 K/ TH
So,300 K/ TH = ¾
TH = 400 K
= (400 -273)° C
= 127 ° C
Therefore the temperature of the high-temperature region TH would be 127 ° C. From the above observation we conclude that, option (C) is correct.
___________________________________________________________________________________________
Problem 10:-
A real has an efficiency of 33%. The engine has a work output of 24 J per cycle.
(a) How much heat energy is extracted from the high temperature reservior per cycle?
(A) 8 J (B) 16 J (C) 48 J (D) 72 J (E) The question can be answered only if the engine is a carnot engine
(b) How much heat energy is discharged into the low-temperature reservior per cycle?
(A) 8 J (B) 16 J (C) 48 J (D) 72 J (E) The question can be answered only if the engine is a carnot engine
(c) For this engine TL = 27ºC, What can be concluded about TH?
(A) TH = 450ºC (B) TH = 177ºC
(C) TH > 177ºC (D) TH < 177ºC
(E) 177ºC < TH < 450ºC
Solution:-
(a) The correct option is (D) 72 J.
The efficiency ε of a Carnot engine is defined as,
ε = |QH| -|QL|/|QH|
=1- |QL|/|QH|
= 1- TL/TH
Here QL is the heat at lower temperature, QH is the heat at higher temperature, TL is the lower temperature and TH is the higher temperature.
As, ε = |QH| -|QL|/|QH|
= ΔW/|QH| (Since, |QH| -|QL| = ΔW)
To obtain the amount of heat energy is extracted from the higher temperature |QH|, substitute 33% for ε and 24 J for ΔW in the equation ε = ΔW/|QH|, we get,
ε = ΔW/|QH|
33/100 = 24 J/|QH|
Or, |QH| = (2400/33) J
= 72.73 J
Rounding off to two significant figures, the amount of heat energy is extracted from the higher temperature |QH| would be 72 J. From the above observation we conclude that, option (D) is correct.
To obtain the heat energy is discharged into the low-temperature reservoir per cycle |QL|, substitute 72 J for |QH| and 33% for ε in the equation ε =1- |QL|/|QH|,
ε =1- |QL|/|QH|
33/100 = 1-|QL|/72 J
|QL|/72 J = 1-33/100
= 67/100
So,|QL| = (67/100)×72 J
= 48.24 J
Rounding off to two significant figures, the heat energy is discharged into the low-temperature reservoir per cycle |QL| would be 48 J. From the above observation we conclude that, option (C) is correct.
To obtain TH, substitute 33% for ε, 27 ° C for TL in the equation ε =1- TL/TH we get,
33/100 =1- TL/TH
= 1-27 ° C/ TH
27 ° C/ TH =1-33/100
=67/100
TH =27 ° C×100/67
=40.30 ° C
<177 ° C
Therefore the value of TH will be <177 ° C. From the above observation we conclude that, option (D) is correct.
__________________________________________________________________________________
Problem 11:-
A Carnot engine discharges 3 J of heat into the low temperature reservior for every 2 J of work output.
(a) What is the efficiency of this Carnot engine?
(A) 1/3 (B) 2/5 (C) 3/5 (D) 2/3
(b) For this engine TL = 27ºC, What can be concluded about TH?
(A) TH = 627ºC (B) TH = 227ºC
(C) TH > 627ºC (D) TH < 227ºC
(E) 227ºC < TH < 627ºC
Solution:-
(a) The correct option is (A) 1/3.
The efficiency ε of a Carnot engine is defined as,
ε = 1- |QL/QH|
= 1- TL/TH
Here QL is the heat at lower temperature, QH is the heat at higher temperature, TL is the lower temperature and TH is the higher temperature.
To obtain the efficiency ε of this Carnot engine, substitute 2 J for QL and 3 J for QH in the equation ε = 1- |QL/QH|,
ε = 1- |QL/QH|
= 1-2 J/3 J
= 1/3
Thus the efficiency of this Carnot engine would be 1/3. From the above observation we conclude that, option (A) is correct.
The efficiency ε of a Carnot engine is defined as,
ε = 1- |QL/QH|
= 1- TL/TH
Here QL is the heat at lower temperature, QH is the heat at higher temperature, TL is the lower temperature and TH is the higher temperature.
To obtain the efficiency ε of this Carnot engine, substitute 2 J for QL and 3 J for QH in the equation ε = 1- |QL/QH|,
ε = 1- |QL/QH|
= 1-2 J/3 J
= 1/3
Thus the efficiency of this Carnot engine would be 1/3.
To obtain TH, substitute 1/3 for ε, 27 ° C for TL in the equation ε =1- TL/TH we get,
1/3=1- TL/TH
= 1-27 ° C/ TH
TH = 27 ° C×3/2
=40.5° C
<227 ° C
Therefore the value of TH will be <227 ° C. From the above observation we conclude that, option (D) is correct.
___________________________________________________________________________________
Problem 12:-
Which of the following is a consequence of the second law of thermodynamics?
(A) Heat can flow only from high temperature to low temperature.
(B) Objects in contact will tend toweard having the same temperature.
(C) Any system that produces order from disorder must have
Solution:-
The correct option is (C).
In accordance to second law of thermodynamics, when changes occur due to external influence within a closed system its entropy either increases (for irreversible process) or remains constant (for reversible process). It never decreases. Entropy is often associated with disorder and the second law of thermodynamics is sometimes cast as a statement that the disorder of a closed system always increases. The final state, with the randomly directed motions of its molecules, is more disorder than the initial state, with the directed motions of a substantially large number of its molecules. Thus any system that produces order from disorder must have an external influence. Therefore option (C) is correct.
______________________________________________________________________________________________
Problem 13:-
A block of aluminum originally at 80º C is placed into an insulated container of water originally at 25ºC. After a while the system reaches an equilibrium temperature of 31ºC.
(a) During the process
(A) ΔSaluminum > 0 (B) ΔSaluminum (C) ΔSaluminum < 0
(b) During this process
(A) ΔSwater > 0 (B) ΔSwater = 0 (C) ΔSwater < 0
(c) During this process
(A) |ΔSwater| > |ΔSaluminum|
(B) |ΔSwater| = |ΔSaluminum|
(C) |ΔSwater| < |ΔSaluminum|
Solution:-
(a) The correct option is (C) ΔSaluminum<0.
Entropy change (ΔS) for a reversible process is defined as,
ΔS = ΔQ/T
Where, ΔQ is the change in heat energy that is transferred into or out of the closed system at constant temperature T.
Whenever an irreversible process takes place, certain amount of energy which could have been utilized for doing useful work changes to a form in which it becomes unavailable. Entropy is a measure of disorder. Thus with disorder, entropy increases. The initial temperature of the aluminum block is 80°C. But after some time the system reaches an equilibrium temperature of 31°C. Therefore the aluminum block will colder than its original state. This signifies that, the change in entropy of the block of aluminum will be less than zero (ΔSaluminum<0). From the above observation we conclude that option (C) is correct.
(b) The correct option is (A) ΔSwater>0.
Entropy change (ΔS) for a reversible process is defined as,
ΔS = ΔQ/T
Where, ΔQ is the change in heat energy that is transferred into or out of the closed system at constant temperature T.
Whenever an irreversible process takes place, certain amount of energy which could have been utilized for doing useful work changes to a form in which it becomes unavailable. Entropy is a measure of disorder. Thus with disorder, entropy increases. The initial temperature of water is 25° C. But after some time the system reaches an equilibrium temperature of 31°C. Therefore the water will be much hotter than its original state. This signifies that, the change in entropy of the water will be greater than zero (ΔSwater>0). From the above observation we conclude that option (A) is correct.
(c) The correct option is (A) |ΔSwater| > | ΔSaluminum|
In accordance to second law of thermodynamics, when changes occur within a closed system its entropy increases for an irreversible process. To satisfy the second law of thermodynamics in the system, the net change in entropy of water must be greater than the net change in entropy of aluminum (|ΔSwater| > | ΔSaluminum|). From the above observation we conclude that, option (A) is correct.
_________________________________________________________________________________
Problem 14:-
For which of the following process is the entropy change zero?
(A) Isobaric (B) Isothermal
(C) Adiabatic (D) Constant volume
(E) None of these, since ΔS > 0 for all process.
Solution:-
The correct option is (C) adiabatic.
Entropy change (ΔS) for a reversible process is defined as,
ΔS = ΔQ/T
Where, ΔQ is the change in heat energy that is transferred into or out of the closed system at constant temperature T.
From equation ΔS = ΔQ/T, the entropy change will be zero when
ΔQ/T = 0
If the above condition is satisfied, ΔQ =0 as T is constant.
This shows that the change in the entropy is constant.
So, the process will be adiabatic process as the amount of heat remains constant. Thus in an adiabatic process the entropy change will be zero. Therefore option (C) is correct.
_____________________________________________________________________________
Problem 15:-
Ten identical particles are to be divided up into two containers.
(a) How many microstates belong to the configuration of three particles in one container and seven in the other?
(A) 120 (B) 30240 (C) 3628800 (D) 6.3 109
109
(b) How many different configuration are possible?
(A) 1 (B) 11 (C) 120 (D) 1024 (E) 3628800
(c) What is the total number of microstates for the ten-particle system?
(A) 1 (B) 11 (C) 120 (D) 1024 (E) 3628800
(d) Which configuration has the largest number of microstates?
(A) 0, 10 (B) 3, 7 (C) 4,6 (D) 5, 5
Solution:-
(a) The correct option is (A) 120.
The number of microstates that lead to a given configuration the multiplicity w of that configuration is defined as,
w = N!/N1! N2!
Here N = 10, N1 = 3 and N2 = 7.
So, w = N!/N1! N2!
= 10!/3! 7!
= 120
From the above observation we conclude that, the microstates belong to the configuration of three particles in one container and seven in other will be 120. Therefore option (A) is correct.
Ten identical particles are to be divided up into two containers. The total number of possible different configuration will be equal to N!.
Here N is the total number of particles.
As N = 10, N! = 10! = 3628800
From the above observation we conclude that, 3628800 different configuration are possible. Therefore option (E) is correct.
Ten identical particles are to be divided up into two containers. The total number of microstates for the ten particle system will be equal to 2N.
Here N is the total number of particles.
As N = 10, 2N = 210 = 1024
From the above observation we conclude that, the total number of microstates for the ten particle system will be 1024. Therefore option (D) is correct.
The number of microstates that lead to a given configuration the multiplicity w of that configuration is defined as,
w = N!/N1! N2!
In the configuration (0,10) , N = 10, N1 = 0 and N2 = 10.
So, w = N!/N1! N2! = 10!/0! 10! = 1
In the configuration (3,7) , N = 10, N1 = 3 and N2 = 7.
So, w = N!/N1! N2! = 10!/3! 7! = 120
In the configuration (4,6) , N = 10, N1 = 4 and N2 = 6.
So, w = N!/N1! N2! = 10!/4! 6! = 210
In the configuration (5,5) , N = 10, N1 = 5 and N2 = 5.
So, w = N!/N1! N2! = 10!/5! 5! = 252
From the above observation we conclude that, the configuration which has the largest number of microstates will be (5,5). Therefore option (D) is correct.
|
Question 1:-A system is taken from state A to state B along two different paths 1 and 2. The heat absorbed and work done by the system along these paths are Q1 and Q2 and W1 and W2respectively. Then
(A) Q1 = Q2 (B) W1 + Q1 = Q2 + W2 (C) W1 = W2 (D) Q1 - W1 = Q2 - W2 Question 2:-When the gas expands under adiabatic condition, its temperature: (a) increases (b) decreases (c) does not change (d) none of the above Question 3:-In a reversible adiabatic change ΔS is (A) infinity (B) zero (C) equal to CvdT (D) equal to nRln V2/V1 Question 4:-If the temperature of source increases, the efficiency of a Carnot’s engine: (A) decreases (B) increases (C) remains the same (D) may increase or decrease Question 5:-If the temperature of the sink is decreased, the efficiency of a Carnot engine: (A) decreases (B) increases (C) remains the same (D) first increase and then decrease Question 6:-Heat cannot flow by itself from a colder body to hotter body is a statement of: (A) 2nd law of thermodynamics (B) conservation of momentum (C) conservation of mass (d) first law of thermodynamics |

| Q.1 | Q.2 | Q.3 | Q.4 | Q.5 | Q.6 |
|
D |
B |
B |
B |
B |
A |
Related Resources:-
-
You might like to refer Solved problems on specific heat, latent heat and entropy.
-
For getting an idea of the type of questions asked, refer the Previous Year Question Papers.
-
Click here to refer the most Useful Books of Physics.
To read more, Buy study materials of Thermodynamics comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

