Covalent Bonding and Valence Bond Theory
Table of Content |
-
Covalancy
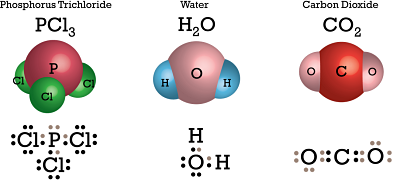
 This type of valency involves sharing of electrons between the concerned atoms to attain the octet configuration with the sharing pair being contributed by both species equally. The atoms are then held by this common pair of electrons acting as a bond, known as covalent bond. If two atoms share more than one pair then multiple bonds are formed. Some examples of covalent bonds are
This type of valency involves sharing of electrons between the concerned atoms to attain the octet configuration with the sharing pair being contributed by both species equally. The atoms are then held by this common pair of electrons acting as a bond, known as covalent bond. If two atoms share more than one pair then multiple bonds are formed. Some examples of covalent bonds are
Refer to the following video for covalent bonding
-
Valence Bond Theory (VBT)
A covalent bond is formed by overlapping of valence shell atomic orbitals of the two atoms having unpaired electron. As a result of overlapping, there is maximum electron density between the bonding atoms and large part of bonding force arises due to electrostatic force of attraction between accumulated electron cloud and two nuclei. Greater the overlapping of atomic orbitals higher is the strength of chemical bond. The paired electron of valence shell of an atom can take part in covalent bonding subject to availability of vacant orbitals of slightly higher energy of the same main energy shell and availability of energy for unpairing of paired electron and their shifting to vacant orbitals. This point explains the trivalency of boron, tetravalency of carbon, pentavalency of phosphorous hexavalency of S and hepta valency of Cl, Br, I.
Depending on type of overlapping atomic orbitals covalent bond can be classified into two types
1. Sigma (s) 2. Pi (p) bond
-
Sigma and Pi Bonding
When two hydrogen atoms form a bond, their atomic orbitals overlap to produce a greater density of electron cloud along the line connecting the two nuclei. In the simplified representations of the formation of H2O and NH3 molecules, the O—H and N—H bonds are also formed in a similar manner, the bonding electron cloud having its maximum density on the lines connecting the two nuclei. Such bonds are called sigma bonds (σ-bond).
A covalent bond established between two atoms having the maximum density of the electron cloud along the line connecting the centre of the bonded atoms is called a σ-bond. A σ-bond is thus said to possess a cylindrical symmetry along the internuclear axis.
Let us now consider the combination of two nitrogen atoms. Of the three singly occupied p-orbitals in each, only one p-orbital from each nitrogen (say, the px may undergo “head –on” overlap to form a s-bond. The other two p-orbitals on each can no longer enter into a direct overlap. But each p-orbital may undergo lateral overlap with the corresponding p-orbital on the neighbour atom. Thus we have two additional overlaps, one by the two py orbitals, and the other by the two pz orbitals. These overlaps are different from the type of overlap in as-bond.
For each set of p-orbitals, the overlap results in accumulation of charge cloud on two sides of the internuclear axis. The bonding electron cloud does no more posses an axial symmetry as with the s-bond; instead, it possess a plane of symmetry. For the overlap of the pz atomic orbital, the xy plane provides this plane of symmetry; for the overlap of the pyatomic orbitals, the zx plane serves the purpose. Bonds arising out of such orientation of the bonding electron cloud are designated as π-bonds.
The bond formed by lateral overlap of two atomic orbitals having maximum overlapping on both sides of the line connecting the centres of the atoms is called a π-bond. A π-bond possess a plane of symmetry, often referred to as the nodal plane.

σ-Bond : When covalent bond is formed by overlapping of atomic orbitals along the same axis it is called s - bond. Such type of bond is symmetrical about the line joining the two nuclei e.g.
|
(a) s-s overlapping
|
|
|
(b) s-p overlapping
|
|
|
(c) p-p overlapping
|
|
π - Bond: This type of bond is formed by the sidewise or lateral overlapping of two half filled atomic orbitals.

|The strength of a bond depends upon the extent of overlapping of half-filled atomic orbitals. The extent of overlapping is between two atoms is always greater when there is end to end overlapping of orbitals than, when there is sidewise overlapping of oritals. Hence s-bond is always stronger than p-bond.
The average distance between the nuclei of the two bonded atoms in a molecule is called bond length and the energy required to break one mole of bonds of particular type in gaseous state is called Bond energy or Bond strength. The same amount of energy is released in formation of one mol of particular bond.
Limitation: VBT cannot explain the paramagnetic properties of B2,O2 etc.
-
Co-ordinate Covalency
A covalent bond results from the sharing of pair of electrons between two atoms where each atom contributes one electron to the bond. It is also possible to have an electron pair bond where both electrons originate from one atom and none from the other. Such bonds are called coordinate bond or dative bonds. Since in coordinate bonds two electrons are shared by two atoms, they differ from normal covalent-bond only in the way they are formed and once formed they are identical to normal covalent –bond.
It is represented as [——→]
Atom/ion/molecule donating electron pair is called Donor or Lewis base. Atom / ion / molecule accepting electron pair is called Acceptor or Lewis acid, [——→] points donor to acceptor
NH4+, NH3 has three (N – H) bond & one lone pair on N – atom. In NH4+ formation this lone pair is donated to H+ (having no electron) NH3 + H+ → NH4+

Properties of the coordinate compounds are intermediates of ionic and covalent compounds.
-
The electronegativity deference between the two atoms should be less.
-
The covalent bond is formed between the two non metals.
-
Each combining atom must contribute at least one electron to the shared pair.
-
The combining atoms should attain the noble gas configuration after bond formation.
-
Polar and Non-Polar Covalent Bonds
Covalent bonds can be classified into following two groups depending on the electronegativity difference between the bonded atoms..
-
Polar covalent bond.
-
Nonpolar covalent bond.
Polar covalent bond is formed between two atoms which have large difference in electronegativity. The electronegativity difference disturbs the distribution of shared pair of electrons between the two atoms as the electron density would be more toward the element which is more electronegative. This will develop partial positive charge on more electronegative element and partial positive charge on less electronegative one. For example, bond between H and F would be polar covalent bond.

 Non-polar covalent bonds are formed between two like atoms i.e. the atoms which have almost same electronegativity. Due to almost same electronegativity, both atoms attract electron pair equally and no charge appears on any atom, and the whole molecule becomes neutral. For example bond between two H atoms would be non-polar.
Non-polar covalent bonds are formed between two like atoms i.e. the atoms which have almost same electronegativity. Due to almost same electronegativity, both atoms attract electron pair equally and no charge appears on any atom, and the whole molecule becomes neutral. For example bond between two H atoms would be non-polar.-
Maximum Covalency
Elements which have vacant d-orbital can expand their octet by transferring electrons, which arise after unpairing, to these vacant d-orbital e.g. in sulphur.

In excited state sulphur has six unpaired electrons and shows a valency of six e.g. in SF6. Thus an element can show a maximum covalency equal to its group number e.g. chlorine shows maximum covalency of seven.
-
Dipole Moment
Difference in polarities of bonds is expressed on a numerical scale. The polarity of a molecule is indicated in terms of dipole moment (μ). To measure dipole moment, a sample of the substance is placed between two electrically charged plates. Polar molecules orient themselves in the electric field causing the measured voltage between the plates to change.
The dipole moment is defined as the product of the distance separating charges of equal magnitude and opposite sign, with the magnitude of the charge. The distance between the positive and negative centres called the bond length.
Thus, = μ = electric charge × bond length = q × d
As q is in the order of 10–10 esu and d is in the order of 10–8 cm, μ is the order of10–18 esu cm. Dipole moment is measured in ‘Debye’ unit (D)
1D = 10–18 es cm = 3.33 × 10–30 coulomb metre
Note:
-
Generally as electronegativity difference increase in diatomic molecules, polarity of bond between the atoms increases therefore value of dipole moment increases.
-
Dipole moment is a vector quantity.
.png)
-
A symmetrical molecule is non-polar even though it contains polar bonds. For example, CO2, BF3, CCl4 etc. because summation of all bond moments present in the molecules cancel each other.

-
Unsymmetrical non-linear polyatomic molecules have net value of dipole moment. For example, H2O, CH3OH, NH3 etc.?
Calculation of Resultant Bond Moments

Resultant dipole moment may be calculated using vectorial method.
μ = √μ12 + μ22 + 2μ1μ2 cos θ

when θ = 0 the resultant is maximum μR = μ1 + μ2
when, θ = 180°, the resultant is minimum μR = μ1 ∼ μ2
For example, CO2 has got dipole moment of zero. The structure of CO2 is.This is a highly symmetrical structure with a plane of symmetry passing through the carbon. The bond dipole of C–O is directed towards oxygen as it is the negative end. Here two equal dipoles acting in opposite direction cancel each other and therefore the dipole moment is zero. Similarly dipole moment of CCl4 is zero while that of CHCl3 is non zero. . Explanation is again in geometry of the molecules both CCl4 & CHCl3 have tetrahedral structure but CCl4 is symmetrical while CHCl3 is non-symmetrical.

Due to the symmetrical structure of CCl4 the resultant of bond dipoles comes out to be zero. But in case of CHCl3 it is not possible as the presence of hydrogen introduces some dissymmetry.
You can also Refer to
JEE Physical Chemistry Syllabus
Reference books of Physical Chemistry
To read more, Buy study materials of Chemical Bonding comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

