Carbon and its Compounds CBSE Class 10 Science Notes Chapter 4
Do you know that everything on the Earth contains carbon? Carbon combines with other elements to form new materials or compounds. In fact, carbon can form nearly 10 million compounds in living things! Some major types of carbon compounds are carbohydrates, lipids, proteins, and nucleic acids. In Class 10 Science, students study carbon and its compounds and learn about the various properties of materials formed by carbon. askIITians experts have created topic-wise online revision notes for CBSE Class 10 Science Chapter 4 Carbon and Its Compounds. You can refer to these notes at any time and revise the complete chapter in less than 20 minutes.
Revision notes of CBSE Class 10 Science Carbon and Its Compounds include topics like covalent bonds in carbon, saturated and unsaturated carbon compounds, chains, branches, rings, homologous series, the nomenclature of carbon compounds, chemical properties of carbon compounds - combustion, oxidation, addition reaction, ethanol, ethanoic acid, and soaps and detergents. Our pointwise revision notes for this chapter cover all these topics in a clear and concise manner so that you can strengthen your concepts. The notes are based on the latest CBSE syllabus and include all the updated topics for your reference.
Online Revision Notes for CBSE Class 10 Science Chapter 4 Carbon and Its Compounds
Two or more elements combine to form a compound. There are two types of compounds- Organic compounds and Inorganic compounds. Organic compounds are the ones that are made up of carbon and hydrogen.
Covalent Bond
The bond formed by sharing a pair of electrons between two atoms is known as Covalent Bond. Carbon forms covalent bonds. Carbon exists in two forms- as a free state and as a combined state. Freeform of carbon is found in graphite, diamond and fullerene. In the combined state, carbon exists as Carbon-dioxide, Glucose, Sugar etc.
Allotropes of Carbon
Different forms of an element that have the same chemical properties but different physical properties are known as Allotropes. There are three allotropes of carbon- diamond, graphite and fullerene.
Diamond
Diamond exists as a three-dimensional network with strong carbon-carbon covalent bonds. Diamonds are hard in nature with high melting points. It shines in the presence of light and it is a bad conductor of electricity. The most common use of diamonds is in making jewellery. It is also used in cutting and drilling tools.
Graphite
Graphite is made from weak Van der Waal forces. Each carbon atom is bonded with the other three carbon atoms to form hexagonal rings. It serves as a good conductor of heat and electricity. It is used as a dry lubricant for machine parts as well as it is used in lead pencils.
Fullerene
It is a hollow cage that exists in the form of a sphere. Its structure is similar to fullerene. But along with hexagonal rings, sometimes pentagonal or heptagonal rings are also present.

Fig.1 Structure of fullerene
Two Important Properties of Carbon
Catenation and tetravalency are the two important properties of carbon. Catenation is a property of carbon by which carbon atoms can link one another via the covalent bonds and can form long chains, closed rings or branched chains etc. Carbon atoms can be linked by single, double or triple bonds. Carbon has a valency of 4 due to which it is known to have tetravalency. Due to this one carbon atom can bond with other 4 carbon atoms, with other atoms also such as Oxygen, Nitrogen etc.
Hydrocarbons
Compounds that are made up of carbon and hydrogen are known as Hydrocarbons. There are two types of hydrocarbons found - Saturated Hydrocarbons and Unsaturated Hydrocarbons. Saturated Hydrocarbons consist of single bonds between the carbon atoms. For Example, Alkanes. Alkanes are saturated hydrocarbons represented by a formula, CnH2n+2.
Unsaturated Hydrocarbons are the ones with double or triple bonds between the carbon atoms. For Example, Alkenes and Alkynes. Alkenes are represented as CnH2n whereas alkynes are represented as CnH2n-2. Some saturated hydrocarbons and unsaturated hydrocarbons are represented as -

Fig.2. Saturated hydrocarbons

Fig. 3. Unsaturated hydrocarbons
The structure of hydrocarbons can be represented in the form of electron dot structure as well as open structures as shown below-
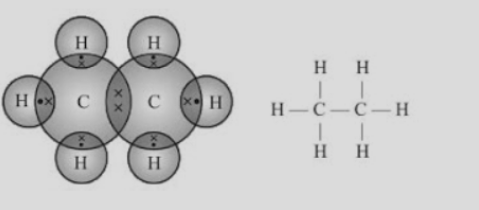
Fig.4. Electron dot structure and open structure of ethane

Fig.5. Electron dot structure and open structure of ethyne
Carbons Compounds based on the structure
Carbon Compounds can be classified as straight-chain compounds, branched-chain compounds and cyclic compounds. They are represented as -
![]()
Fig.6. Straight chain carbon compound
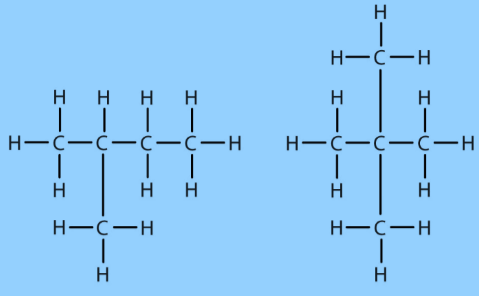
Fig.7. Branched-chain compounds
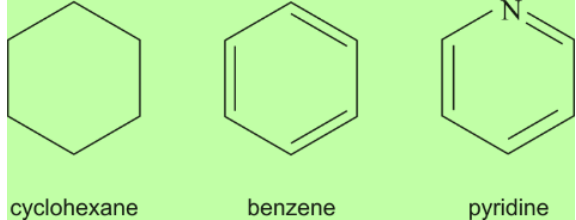
Fig.8. Cyclic carbon compounds
Functional Groups
One of the hydrogen atoms in hydrocarbons can be replaced by other atoms according to their valencies. The atoms which decide the properties of the carbon atoms are known as Functional Groups. For Example, Cl, Br, -OH, Aldehyde, Ketone, Carboxylic Acid etc.
Homologous Series
Series of compounds in which the same functional group substitutes for the hydrogen atom in a chain of carbon.

Fig.9. Homologous series
Nomenclature of Carbon Compounds
- First of all, identify the number of carbon atoms in compounds. And in it identify the longest chain
- Then the functional group can be indicated by suffix or prefix.
- Cyclic hydrocarbon is designated by the prefix cyclo.
- If there are two or more different substituents they are listed in alphabetical order
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given

Fig.10. Different functional groups
Chemical Properties of Carbon Compounds
Combustion
Carbon along with its compound is used as a fuel as it burns in presence of oxygen to release energy. Saturated hydrocarbons produce blue and non-sooty flame whereas unsaturated hydrocarbons produce a yellow sooty flame.
CH4 + 2O2 → CO2 + 2H2O
Oxidation
Alcohol can be oxidised to aldehydes whereas aldehydes, in turn, can be oxidised to carboxylic acid. Oxidising agents such as potassium permanganate can be used for oxidation.

Addition Reaction
Hydrogenation of vegetable oil is an example of an additional reaction. Addition of hydrogen in presence of catalysts such as nickel or palladium. This converts the oil into ghee.

Substitution Reaction
When one atom in hydrocarbon is replaced by chlorine, bromine, etc. this is known as a Substitution Reaction.

Important Carbon Compounds: Ethanol and Ethanoic Acid
Ethanol is a volatile liquid with a low melting point. It reacts with sodium to form sodium ethoxide.

This reaction is used to test the presence of ethanol by the evolution of hydrogen gas.
Dehydration of ethanol in presence of hot sulphuric acid forms alkene.

Ethanoic acid is a colourless liquid. When pure ethanoic acid freezes like ice, it is known as Glacial Acetic Acid. It is formed at a temperature of about 16.6 degrees centigrade
Ethanoic Acid/Acetic acid when reacts with ethanol forms an ester. Ester can be identified by its sweet smell.

The reaction of esters with a strong base is used to form soap. This is known as Saponification. Acetic acid also reacts with a strong base to form sodium acetate and water.
NaOH + CH3COOH + CH3COONa + H2O
Soaps and Detergents
Sodium or potassium salt of a carboxylic acid is known as Soap. They work most effectively in soap water. Detergents are sulphonate or ammonium salt of a long chain of carboxylic acid. They can work effectively on soft as well as hard water.
Cleansing Action of Soaps and Detergents
The cleansing action of soaps and detergents is due to the ability to minimise the surface tension of water, to emulsify oil or grease and hold them in a suspension of water. When soap dissolves in water, it forms soap anions and soap cations. The hydrophobic part of soaps and detergents are soluble in grease and the hydrophilic part is soluble in water.
Soap and Micelle Formation
When dirt and grease are mixed with soap water, soap molecules arrange them in tiny clusters known as Micelle. The hydrophilic part sticks to the water and forms the outer surface of the micelle and the hydrophobic part binds to oil and grease.

CBSE Class 10 Revision Notes for Chapter 4 Carbon and Its Compounds FAQs
- Why study CBSE Class 10 Science Chapter 4 Carbon and Its Compounds?
This chapter helps you understand the versatile nature of carbon. In this chapter, you will study how Carbon forms covalent bonds with itself and other elements such as hydrogen, oxygen, sulphur, nitrogen and chlorine. Also, you will learn different elements made from carbon such as alcohols, aldehydes, ketones, carboxylic acids, soaps and detergents.
- Why are revision notes for Carbon and Its Compounds important?
- The revision notes for Carbon and Its Compounds are based on the latest CBSE syllabus for Class 10 Science and are a perfect resource for exam preparation.
- These notes include pointwise explanations for all the topics of the chapter that will solidify your conceptual understanding.
- The notes are available online for free and can be accessed by the students at any time.
- The notes also include diagrams and tables that will help you memorise the concepts in a better way.
- Is the NCERT book enough to prepare Class 10 Science Chapter 4 Carbon and Its Compounds?
NCERT textbook for Class 10 Science includes easy-to-understand language so that you get a basic understanding of the concepts of carbon and its compounds. The CBSE Class 10 Science board exam is based on the NCERT textbook only. But, if you want to practice a variety of questions based on Carbon and Its compounds you would need additional study materials.
- How can askIITians help me in preparing CBSE Class 10 Science Chapter 4 Carbon and Its Compounds?
At askIITians, we provide different study resources for Class 10 Science that will help you prepare the Chapter Carbon and Its Compounds thoroughly. These study materials include chapter-wise revision notes, NCERT solutions, extra questions, daily practice papers, previous year question papers and much more. We also provide Class 6, 7, 8, 9, 10, 11, 12 notes for Science and Maths to help students prepare every concept thoroughly.
- How online classes for CBSE Class 10 Science can help me excel in my studies?
askIITians’ online CBSE coaching for Class 10 Science includes online live classes for students where our experts teach every concept of Class 10 Science. Their innovative teaching methodologies help students master every concept with ease.